Cryptococcus siRNAs¶
Cryptococcus is one of the few fungi that can become virulent, especially in immunocompromised individuals. Because this fungus has the capacity to grow at temperatures of around 37oC it has been proposed that, apart from a developed immune system, sustaining a high body temperature prevents fungal invasion [Casadevall-2020].
One of the driving forces to develop Coalispr was to create an alternative tool for counting RNA-Seq data obtained for siRNA libraries. We had generated a large number (>100) of such libraries for Cryptococcus deneoformans JEC21 (see About). HTSEQ, the commonly used program to obtain read-counts, required the assembly of an annotation file for siRNA-targets to direct the counting. Small non-coding RNAs have not been annotated, so this had to be done from scratch. A GTF was prepared by manually scanning bedgraph traces in a genome browser like IGB and copying genome coordinates over to a text file and name these regions. From this file a GTF was constructed with a feature useful for counting. Apart from being laborious, this method is error-prone and not very scalable to other organisms or when an update of the genome is published. The counting was also very time-consuming at the time.
This tutorial is based around published data for the related fungus, C. neoformans H99 [Dumesic-2013], [Burke-2019]. As shown below, it can be demonstrated that exons in transcripts, rather than introns [Dumesic-2013], are the target of the RNAi machinery. In C. deneoformans JEC21, siRNAs also align to exons and for both fungi siRNAs crossing splice junctions are identified after mapping with STAR, e.g. at locus CNAG_03231. These findings demonstrate that RNAi acts on spliced transcripts, thus downstream from (instead of parallel to) splicing.
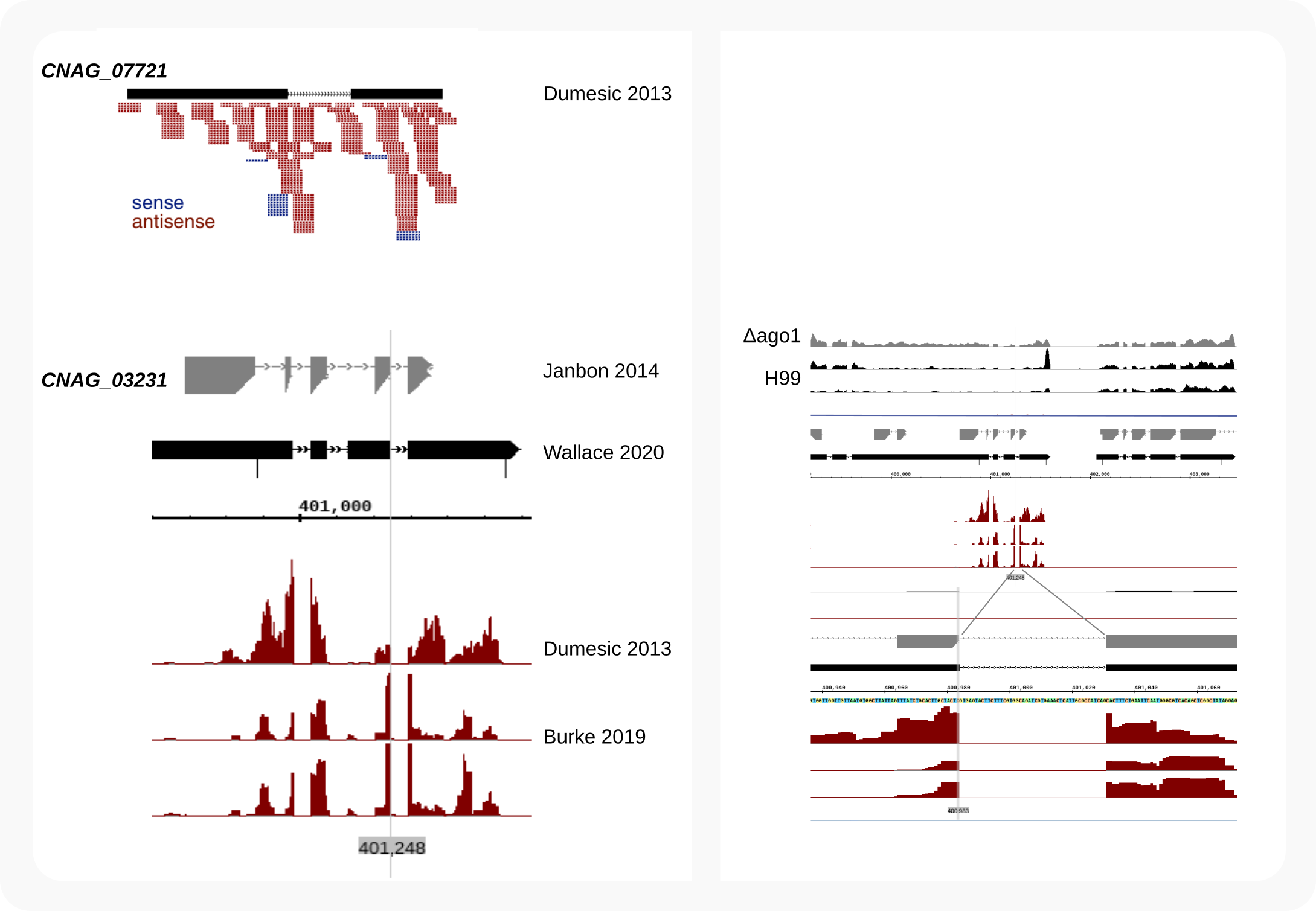
Figure 1. C. neoformans siRNAs target exons, not introns¶
Compared to other eukaryotes, and possibly related to their ability to grow at 37oC, the GC-content in C. (de)neoformans is very high (~48%). Almost all transcripts carry introns (~98%), often about 5-6 per gene, which appear essential for stable expression [Goebbels-2013], [Janbon-2018]. These unique characteristics [1] make it difficult to predict genes and splicing patterns, which we have encountered when designing tagging strategies. This might also be the reason for the inaccurate model Dumesic et al. put forward in their seminal paper of 2013 [Dumesic-2013]. They proposed that in C. neoformans H99 a pre-mRNA was either spliced or redirected to the RNAi machinery, which was based on the observation that many siRNAs mapped to introns. The annotation file they had used must have been a premature version, as almost all siRNAs actually align to exon sequences described in GTFs published since, including those [2] for the original genomic sequence of this fungus [Janbon-2014]. For example, one of their signature genes, CNAG_07721, has been re-annotated, with the chromosome 8 locus [3] renamed to CNAG_03231.
Bedgraphs, count data accumulated with HTSEQ and hand-made annotations for siRNA loci, supported development of Coalispr and the creation of images for the ‘How-to guides’. This tutorial will show how these images were made. The data will be from publications [Burke-2019] and [Dumesic-2013] which analyzed various genes for their contribution to siRNA accumulation after their disruption with NAT or HYG ([Burke-2019]).
Disrupted Factor |
Reference |
Gene::Marker |
Chromosome |
Strand |
Coordinates |
Short |
|---|---|---|---|---|---|---|
Ago1 |
CNAG_04609::NATR |
|
plus |
|
|
|
Rdp1 |
CNAG_03466::NATR |
|
minus |
|
|
|
Rde1 |
CNAG_01848::NATR |
|
minus |
|
|
|
Rde2 |
CNAG_04954::NATR |
|
plus |
|
|
|
Rde3 |
CNAG_06643::NATR |
|
plus |
|
|
|
Rde4 |
CNAG_01157::NATR |
|
minus |
|
|
|
Rde5 |
CNAG_04791::NATR |
|
plus |
|
|
|
Rrp6 |
CNAG_02773::NATR |
|
minus |
|
|
|
Clr4 |
CNAG_05404_Cterm∆::HYG |
|
plus |
|
|
|
Ezh2 |
CNAG_07553::NATR |
|
minus |
|
|
The organization of reference files will be different from that in the yeast and mouse tutorials. Most downloaded and processed references used for the running of Coalispr are placed in a subfolder, source/, of the work directory.
Note
Outcome of below data-preparation can be downloaded from zenodo.org. See/skip to here
First,
- create a work directory (say, Burke-2019/)
Then, move some files that have been shipped with the program:
copy the contents of this folder in the source distribution: coalispr/docs/_source/tutorials/H99/shared/ to
Burke-2019/.
In a terminal, change directory to the created work environment (from which all scripts and commands will be run):
cd /<path to>/Burke-2019/
Dataset¶
Following the mouse and yeast tutorials we first generate bedgraph files for C. neoformans data that has been collapsed and uncollapsed. We can obtain bedgraphs after aligning the data to the reference genome. To do this, the raw sequencing data is used and downloaded from the Gene Expression Omnibus (GEO) database with the relevant accession numbers retrieved from the literature:
H99-data |
Reference |
GEO acc. no. |
SRA table |
SRA study |
|---|---|---|---|---|
WT, mutants |
|
|||
WT 2010 |
|
As described for the yeast tutorial:
Open the GEO Accession Display page for the
GSE128009experiment of [Burke-2019].Enter the accession no. into the field
GEO accessionand pressGO.Access
SRA Run Selectorfrom the bottom of the GEO accession display page that has been opened.On the SRA Run Selector webpage, select all
Assay Typelanes formiRNA-Seqand chooseSelectedin theSelectpane.Then click on the
Accession Listbutton in theSelectedrow of theSelectpane.Save the file to the work directory and, because it will be combined with other data, add a prefix.
Save as1_SRR_Acc_List.txt.For detailed info, click the
Metadatabutton for a file describing the experiment.Save asSraRunTable.txt.Do the same for the [Dumesic-2013] sample.
Save theAccession Listfrom theTotalSelectpanel as2_SRR__Acc_List.txt.Save theMetadataas2_SraRunTable.txt.
Collate the lists:
cat 1-SRR_Acc_List.txt 2-SRR_Acc_List.txt > SRR_Acc_List.txt
Download, extract fastq and compress the data (These steps create a directory structure (see Mouse miRNAs) in the working folder the scripts rely on).
sh 0_0-SRAaccess.shThis takes awhile, and so does:sh 0_1-SRAaccess.shsh 0_2-gzip-fastq.sh
Clean and collapse¶
Adapters present on the reads are removed with:
sh 1_0-flexbar-trim.sh
and a fasta file with collapsed reads is prepared with:
sh 2_0-collapse.sh
Reference traces¶
Traces for gene-expression in cells can be included in Coalispr. Comparing siRNA signals to mRNA traces is possible because various RNA-Seq datasets are available. Here, we use the bedgraphs prepared by [Wallace-2020] and their annotation file. The reference files will be saved in subfolders of the folder source in the work directory Burke-2019/:
mkdir source
The mRNA reads for parental strain H99 and deletion strains for GWO1 and AGO1 are at GEO accession no. GSE133125 [Wallace-2020]; other samples - not used here - are at GSE206758 [Freitas-2022].
RNA-Seq |
SRA acc. no. |
Run |
Bedgraph |
|---|---|---|---|
H99r1 |
|
|
|
H99r2 |
|
|
|
HdGWO1 |
|
|
|
HdAGO1 |
|
|
Bedgraphs are downloaded and saved to their own folders; you could create these first:
cd sourcemkdir GSM3900523 GSM3900527 GSM3900525 GSM3900529cd ..
Now, for each experiment get the compressed bedgraph files for the PLUS [4] and MINUS strand from the GSM3900523 to GSM3900529 linked pages and save these, ending up with the following folder-hiearchy:
burke-2019
└── source
├── GSM3900523
| ├── GSM3900523_PN5_index1_ATCACG_L004_R1_001_hisat2_minus.bedgraph.gz
| └── GSM3900523_PN5_index1_ATCACG_L004_R1_001_hisat2_plus.bedgraph.gz
...
└── GSM3900529
├── GSM3900529_PN10_index18_GTCCGC_L008_R1_001_hisat2_minus.bedgraph.gz
└── GSM3900529_PN10_index18_GTCCGC_L008_R1_001_hisat2_plus.bedgraph.gz
Then decompress and clean up the files [5]:
for i in source/GSM39*/GSM39*.bedgraph.gz; do gunzip -c $i | sed 's/chr//g' > ${i%.*}
Alignment¶
Although siRNA reads are expected to be short (~19-24nt) the high density of introns in Cryptococcus makes it feasible that these RNAs can be complementary to regions that cross splice junctions. In other words, it is possible that siRNAs target transcript sections that have undergone splicing. An RNA-focused mapper like STAR can insert gaps that coincide with possible introns when aligning reads to a reference genome. This is the reason why this aligner features in these tutorials.
References¶
For accurate assignment of introns by STAR it is important to use all information available. One of these resources will be the annotation files describing exons for genes. A second resource is a database of possible splice-junctions that STAR accumulates while alligning reads in an initial pass. Maybe redundant, but we will use both. When generating a genome index, splice-junctions will be retrieved from a recent annotation file; while those found previously on the fly have been collected in a specific file. This file, SpliceJunctionDataBase-sorted-uniq.txt, informs STAR during alignments made in this tutorial. It will also be shown how this file can be updated.
Genome¶
Download a reference genome for aligning reads to the source/ subfolder:
wget -cN --show-progress -P source "https://ftp.ebi.ac.uk/ensemblgenomes/pub/release-55/fungi/fasta/fungi_basidiomycota1_collection/cryptococcus_neoformans_var_grubii_h99_gca_000149245/dna/Cryptococcus_neoformans_var_grubii_h99_gca_000149245.CNA3.dna_sm.toplevel.fa.gz"
Decompress and simplify seq_id:
gunzip -c source/Cryptococcus_neoformans_var_grubii_h99_gca_000149245.CNA3.dna_sm.toplevel.fa.gz | sed 's/ dna_sm:chromosome [a-z0-9A-Z: ]*$//g' > source/h99.fasta
GTFs¶
A GFF3 file with transcript annotations used by [Wallace-2020] can be obtained from their github repository:
wget -cN --show-progress -P source "https://github.com/ewallace/CryptoTranscriptome2018/raw/master/CnGFF/H99.10p.aATGcorrected.longestmRNA.2019-05-15.RiboCode.WithStart.gff3
The file has the format of a GTF file with gene-id entries, but gene names can be simplified [6]:
sort -k 1n,1n -k 4n,4n -k 5n,5n source/H99.10p.aATGcorrected.longestmRNA.2019-05-15.RiboCode.WithStart.gff3 | sed 's/"CN[A-N]:/"/g' > source/h99.gtf
The file lacks descriptions for the mitochondria which is obtained below.
To get a reference for non-coding RNAs and pseudo transcripts, get the GenBank annotations
wget -cNrnd --show-progress --timestamping -P source "ftp://ftp.ncbi.nlm.nih.gov/genomes/genbank/fungi/Cryptococcus_neoformans/latest_assembly_versions/GCA_000149245.3_CNA3/GCA_000149245.3_CNA3_genomic.gbff.gz"
Copy coalispr/coalispr/resources/share/genbank2gtf.py to the work folder [7] and run:
python3 genbank2gtf.py -f source/GCA_000149245.3_CNA3_genomic.gbff.gz -p /<path to source folder>/coalispr/coalispr/resources/share/conversion_tables -c H99 -k 4 -o source/ncRNA-pseu.gtf
Get descriptions for the
MTchromosome and add these to the general GTF.
python3 genbank2gtf.py -f source/GCA_000149245.3_CNA3_genomic.gbff.gz -p /<path to source folder>/coalispr/coalispr/resources/share/conversion_tables -c H99 -k 3 -o source/mito.gtfcat source/mito.gtf | sed '1,2d' >> source/h99.gtf
A reference file for counting with HTSeq had been prepared by copy pasting IGB information to a tsv file, source/h99-siRNAsegments.tsv from which a GTF with annotations was created for counting specific reads. In this GTF a specific feature (GTFFEAT) characterizes siRNA segments. The file forms a positive-control reference (GTFSPEC) in Coalispr displays of bedgraph traces. To make this GTF, copy coalispr/coalispr/resources/share/gtf_frm_tsv.py to the work directory (Burke-2019/) and run
python3 gtf_frm_tsv.py -f source/h99-siRNAsegments.tsv -c 2The option-c 2indicates that the input file (-f) relates to H99.
Two other files, h99-xtra.gtf and h99-ncRNAxtra.gtf are shipped with the tutorial that contain additions based on:
possible extraneous DNAs added to the genomes of knockout mutants (i.e. marker genes conferring resistance to nourseothricin (NAT), neomycin/geneticin/G418 (G418) or hygromycin (HYG).
observed differences between data traces and downloaded annotations.
These files will be automatically included when referred to (via GTFXTRA, GTFXTRANAM and GTFUNXTR, GTFUNXTNAM) in the settings file 3.h99.txt and loaded with coalispr showgraphs.
Index¶
With the annotation file for intron definitions, we can now generate with STAR a genome index for mapping reads to the reference genome. This creates a new folder, the name of which depends on the provided argument. Best practice is that the name for this folder contains EXP, which is set during coalispr init; here we take h99:
sh 3_0-createSTARindex.sh h99
Note that this file import the fasta file with the sequence for the extra annotations as set in h99-xtra.gtf.
The file with chromosome lengths (LENGTHSNAM), created by STAR, can be linked to; place the symbolic shortcut in source/, with other resources for Coalispr:
cd source; ln -sf ../star-h99/chrNameLength.txt h99-chr-lengths.txt; cd ..
Mapping¶
Map collapsed reads first, this should be fairly quick. Add argument EXP to find the folder with the correct genome information. A second argument would define the number of allowed mismatches; for stringent end-to-end mapping use 0. A third argument can be added to indicate that splice junctions have to be re-recorded from gapped alignments. Set this to 1 if needed; by default this is skipped because such a record comes with the tutorial (also see H99-shared).
sh 3_1-run-star-collapsed-14mer-strict.sh h99 0If splice junction data have been collected [8], prepare the new database with:sh 3_1_1-run-make-SJDB.sh h99
If this all finishes ok, map the uncollapsed data; by default the available database of spliced junctions will be used.
sh 4_1-run-star-uncollapsed-14mer-strict.sh h99[9]
Then, bedgraphs can be made. The scripts take two parameters, for EXP and the number (0 by default) of mismatches.
sh 3_2-run-make-bedgraphs.sh h99sh 4_2-run-make-bedgraphs-uncollapsed.sh h99
For quality control information, the following script (with the same two parameters) is available:
sh 5_1-QC-reports.sh h99
- As a check for the mapping we inspect in a genome browser the accumulation of cDNAs, i.e. the collapsed data, at the CNAG_03231 locus on chromosome 8. The number of sense cDNAs is in a comparable range as the antisense cDNAs, but far more copies of antisense siRNAs are expressed as shown in Figure 1 where the uncollapsed sense signals are not coming above the base line.
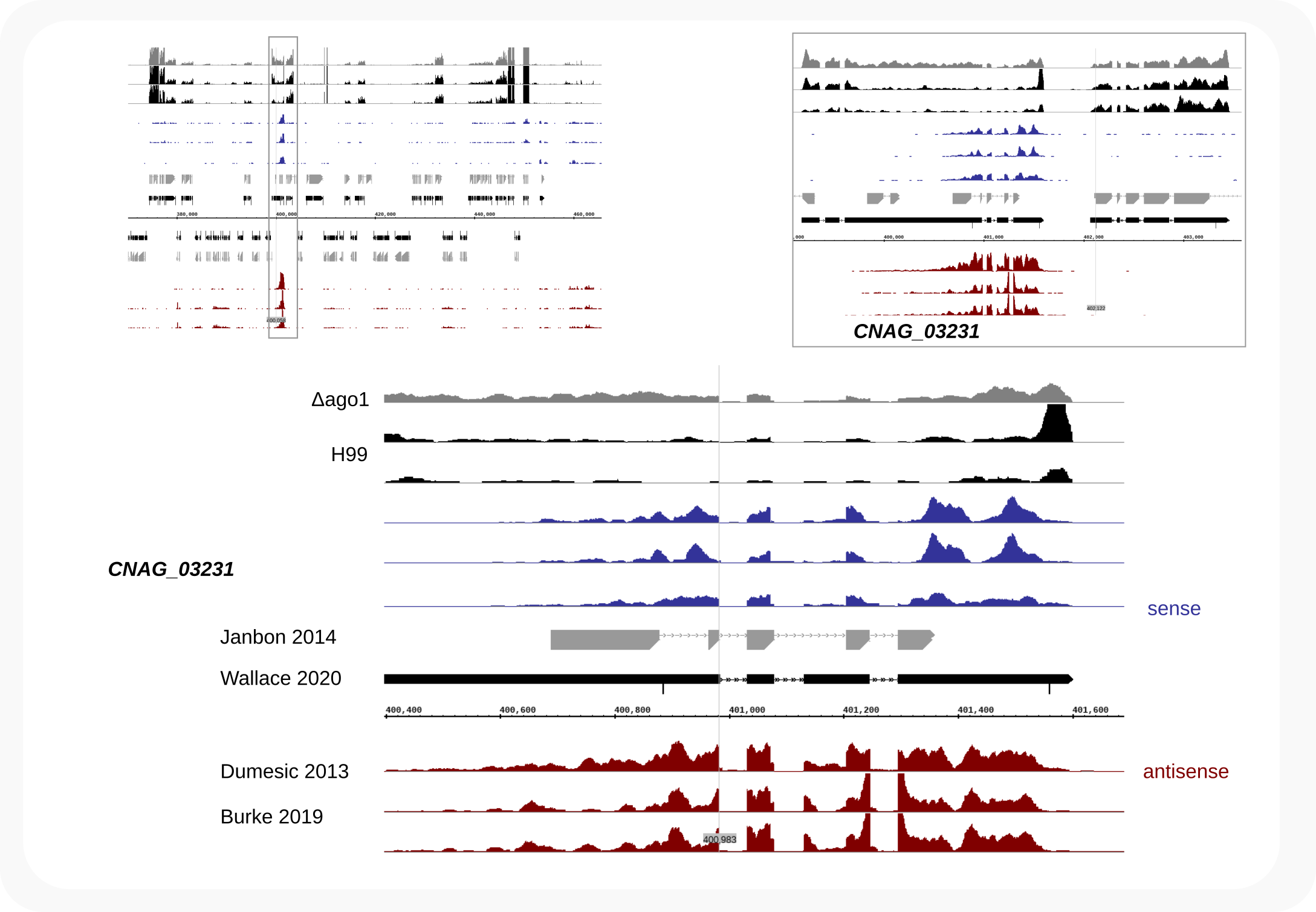
Figure 2. C. neoformans siRNA cDNAs target exons, not introns¶
Note
Datasets prepared above were uploaded to zenodo.org (DOI 10.5281/zenodo.12822543). These can also be used for testing Coalispr.
cd <path to workfolder>wget -cNrnd --show-progress --timestamping "https://zenodo.org/records/12822544/files/Burke-2019.tar.gz?download=1"tar -xvzf Burke-2019.tar.gz
Updated versions of Coalispr may require new configuration files, please check the zenodo site.
Coalispr¶
The input materials, the bedgraph files, are available. The analysis begins with setting up the program for this experiment (EXP), which will be named to h99.
cd /<path to>/Burke-2019coalispr initbash-5.2$ coalispr init Please provide a name for this new session/experiment. This name will be used in commands and in file paths. (therefore best to keep it short and memorable, without spaces or special characters). Experiment name: ('stop' to cancel) h99 Experiment name will be set to: 'h99' Is that OK? 1: Yes 2: Cancel ?: 1 Experiment name has been set to 'h99'. Now, set the work folder for this session/experiment. Files are stored in folder 'Coalispr' either in the user's home directory, or in the current folder (before running the `init` command, change directory to this destination first). Alternatively keep files near the source code, in the coalispr installation folder. (The directory with the installation folder will be shown as option in this case.) Configuration files, figures and data go in sub-folders: 'constant_in' for configuration files, 'figures', and 'data' for storage of .pkl and .tsv count files. Please choose a folder. ('stop' to cancel) 1: home (rob) 2: current (Burke-2019) 3: source (COALISPR) Enter an option: 2 Path to Coalispr folder will be set to: '/<path to>/Burke-2019/Coalispr' Is that OK? 1: Yes 2: Cancel ?: 1 Configuration files to edit are in: '/<path to>/Burke-2019/Coalispr/config/constant_in' The path '/<path to>/Burke-2019/Coalispr' will be set as 'SAVEIN' in 3_h99.txt.
Configuration¶
Explained in the How-to guides is the preparation of configuration files with settings, especially the 3_EXP.txt, and the file (EXPFILE) describing the data for the experiment.
Note
When using the alignments downloaded from zenodo.org, the EXPFILE, /<path to>/Burke-2019/source/h99-experiment_table.tsv, is ready and only the shipped configuration file /<path to>/Burke-2019/Coalispr/constant_in/3_h99_zenodo.txt needs to be adapted to your local setup:
From
3_h99.txtcreated bycoalispr init, copy relevant [12] values for:SETBASE
SAVEIN (BASEDIR / PRGNAM).
and replace those in
3_h99_zenodo.txtRemove/rename
3_h99.txtRename/link
3_h99_zenodo.txtto3_h99.txt
Experiment file¶
The file describing the experiment has been assembled from the SraRunTable.txt for the two GEO datasets as follows:
Short Run Category Method Group Fraction Condition Read-density Experiment Variation GEO_Accession (exp) DOI
wt_0 SRR646636 S total WCE NaN SRX215659 WT 2010 GSM1061025 10.1016/j.cell.2013.01.046
a1_1 SRR8697580 U total a1 WCE NaN SRX5493436 ago1 GSM3660325 10.25387/g3.7829429
a1_2 SRR8697581 U total a1 WCE NaN SRX5493437 ago1 GSM3660326 10.25387/g3.7829429
c4_1 SRR8697582 M total meth WCE NaN SRX5493438 clr4 GSM3660327 10.25387/g3.7829429
c4_2 SRR8697583 M total meth WCE NaN SRX5493439 clr4 GSM3660328 10.25387/g3.7829429
e2_1 SRR8697584 M total meth WCE NaN SRX5493440 ezh2 GSM3660329 10.25387/g3.7829429
e2_2 SRR8697585 M total meth WCE NaN SRX5493441 ezh2 GSM3660330 10.25387/g3.7829429
re1_1 SRR8697586 M total re1 WCE NaN SRX5493442 rde1 GSM3660331 10.25387/g3.7829429
re1_2 SRR8697587 M total re1 WCE NaN SRX5493443 rde1 GSM3660332 10.25387/g3.7829429
re2_1 SRR8697588 M total re2 WCE NaN SRX5493444 rde2 GSM3660333 10.25387/g3.7829429
re2_2 SRR8697589 M total re2 WCE NaN SRX5493445 rde2 GSM3660334 10.25387/g3.7829429
re3_1 SRR8697590 M total re3 WCE NaN SRX5493446 rde3 GSM3660335 10.25387/g3.7829429
re3_2 SRR8697591 M total re3 WCE NaN SRX5493447 rde3 GSM3660336 10.25387/g3.7829429
re4_1 SRR8697592 M total re4 WCE NaN SRX5493448 rde4 GSM3660337 10.25387/g3.7829429
re4_2 SRR8697593 M total re4 WCE NaN SRX5493449 rde4 GSM3660338 10.25387/g3.7829429
re5_1 SRR8697594 M total re5 WCE NaN SRX5493450 rde5 GSM3660339 10.25387/g3.7829429
re5_2 SRR8697595 M total re5 WCE NaN SRX5493451 rde5 GSM3660340 10.25387/g3.7829429
r1_1 SRR8697596 U total r1 WCE NaN SRX5493452 rdp1 GSM3660341 10.25387/g3.7829429
r1_2 SRR8697597 U total r1 WCE NaN SRX5493453 rdp1 GSM3660342 10.25387/g3.7829429
r6_1 SRR8697598 M total r6 WCE NaN SRX5493454 rrp6 GSM3660343 10.25387/g3.7829429
r6_2 SRR8697599 M total r6 WCE NaN SRX5493455 rrp6 GSM3660344 10.25387/g3.7829429
wt_1 SRR8697600 S total WCE NaN SRX5493456 Kn99alpha GSM3660345 10.25387/g3.7829429
wt_2 SRR8697601 S total WCE NaN SRX5493457 Kn99alpha GSM3660346 10.25387/g3.7829429
h99_1 GSM3900523 R rnaseq 1 SRX6102296 RNA_H99r1 GSM3900523 10.1093/nar/gkaa060
h99_2 GSM3900527 R rnaseq 4 SRX6102300 RNA_H99r2 GSM3900527 10.1093/nar/gkaa060
h99-a1 GSM3900529 R rnaseq 5 SRX6102302 RNA_HdAGO1 GSM3900529 10.1093/nar/gkaa060
h99-g1 GSM3900525 R rnaseq 2 SRX6102298 RNA_HdGWO1 GSM3900525 10.1093/nar/gkaa060
For columns METHOD, FRACTION or GROUP only values relating to particular methods, fractions or conditions are filled in. When one value applies to all samples it can be omitted; but then the group will not be shown in coalispr showgraphs. Thus, in the example above, METHOD and FRACTION will be visible but CONDITION will not be indicated. The numbers in the Read-density (READDENS) column for the mRNA references lead to an adaptation of the traces so that their overall display is at a comparable level. This can be done because the expression of most transcripts do not seem to be affected by the deletions. In other words, it is an application of the principle guiding normalization when differential expression of genes is assessed (see About) [10]. Adjusting the relative Read-density makes it easier to observe putative effects of the absence of Ago1 or Gwo1 on expression of transcripts targeted by siRNAs.
Settings file¶
For the configuration, in the file 3_h99.txt (copied from 3_EXP.txt in the init step) the following fields [11] were set to the given values:
EXP : “h99” [12]
CONFNAM : “3_h99.txt” [12]
EXPNAM : “C. neoformans”
UNSPECLOG10 : 0.78
MAXGAP : 400
XLIM0 : 14
XLIM1 : 36
XLIM00 : 18
SETBASE : “/<path to>/Burke-2019/”
REFS : SOURCE
EXPFILNAM : “h99-experiment_table.tsv”
RIP1 : “”
RIP2 : “”
NOTAGIP : “”
- MUTGROUPS{
- “a1”:”ago1\u0394”, “re1”:”rde1\u0394”, “re2”:”rde2\u0394” [13]“re3”:”rde3\u0394”, “re4”:”rde4\u0394”, “re5”:”rde5\u0394”,“r1”:”rdp1\u0394”, “r6”:”rrp6\u0394”, OTHR:”methyl\u0394”,}
- FRACTIONS{
- WCE:”Cell extract”,}
- UNSPECIFICS[
- “a1”, “r1”,]
- MUTANTS[
- “re1”, “re2”, “re3”, “re4”, “re5”, “r6”, OTHR,]
LENGTHSNAM : “h99-chr-lengths.txt”
DNAXTRNAM : “NAT_G418_HYG.fa”
LENXTRA : “7062”
CHRXTRA : XTRA
GTFSPECNAM : “h99-siRNAsegments.gtf”
GTFUNSPNAM : “ncRNA-pseu.gtf”
GTFREFNAM : “h99.gtf”
GTFXTRANAM : “h99-xtra.gtf”
GTFUNXTNAM : “h99-ncRNAxtra.gtf”
# SAVEIN : Path(“/<path to>/Burke-2019/Coalispr”) [12]
SAVEIN : BASEDIR / PRGNAM [14]LENGTHSFILE : BASEDIR / SOURCE / LENGTHSNAM
DNAXTRA : BASEDIR / SOURCE / DNAXTRNAM
EXPFILE : BASEDIR / SOURCE / EXPFILNAM
GTFSPEC : BASEDIR / SOURCE / GTFSPECNAM
GTFUNSP : BASEDIR / SOURCE / GTFUNSPNAM
GTFREF : BASEDIR / SOURCE / GTFREFNAM
GTFXTRA : BASEDIR / SOURCE / GTFXTRANAM
GTFUNXTR : BASEDIR / SOURCE / GTFUNXTNAM
Analysis¶
Bedgraph data are loaded and compared in Coalispr by means of Pandas dataframes. For repeated use, the data are stored in a binary (pickle) format specific for the version of Python used to run Coalispr. The coalispr storedata steps below accomplish this.
Note
When using the archive with alignments downloaded from zenodo.org, this step can be skipped and binary, processed data that is used in Coalispr can be obtained with coalispr storedata -d4 after which subsequent commands (like coalispr showgraphs or coalispr countbams) can be tested. After this, or when generating your own data files through coalispr storedata -d{1,2} -t{1,2} remove or rename the backup_from_pickled link (to prevent that the contents shipped with the archive are overwritten by subsequent coalispr storedata -d3 commands).
Store uncollapsed, then collapsed data (-t2), while processing both data (-d1) and reference (-d2) bedgraph files.
coalispr storedata -d1coalispr storedata -d2coalispr storedata -d1 -t2coalispr storedata -d2 -t2
Example output of last run, skipping files already processed.
bash-5.2$ coalispr storedata -d2 -t2
Processing references ...
Binned bedgraph-frames available for 'h99-a1'; will be skipped
Binned bedgraph-frames available for 'h99_1'; will be skipped
Binned bedgraph-frames available for 'h99_2'; will be skipped
Binned bedgraph-frames available for 'h99-g1'; will be skipped
Nothing left to bin, skip this.
Pickle-file for 'reference_merged' saved
Merged references to filter: ['h99_1', 'h99_2', 'h99-a1', 'h99-g1']
Using ['a1_2', 'r1_2', 'a1_1', 'r1_1'] for removing aspecific signals from references
Pickle-file for 'reference_specific_0-78_collapsed' saved
Pickle-file for 'reference_unspecific_0-78_collapsed' saved
The outputs written to the terminal also end up in the log file in /Burke-2019/Coalispr/logs/h99/. Check the logs to get an idea about the involved steps, whereby contiguous indices for regions with signals are combined to segments. Further, files are saved for use by other parts of the program based on the threshold UNSPECLOG10 (of 0.78; representing a minimal ~6 fold difference in the case of overlapping signals for SPECIFIC/MUTANT samples vs. UNSPECIFIC samples in bins marked by the indices).
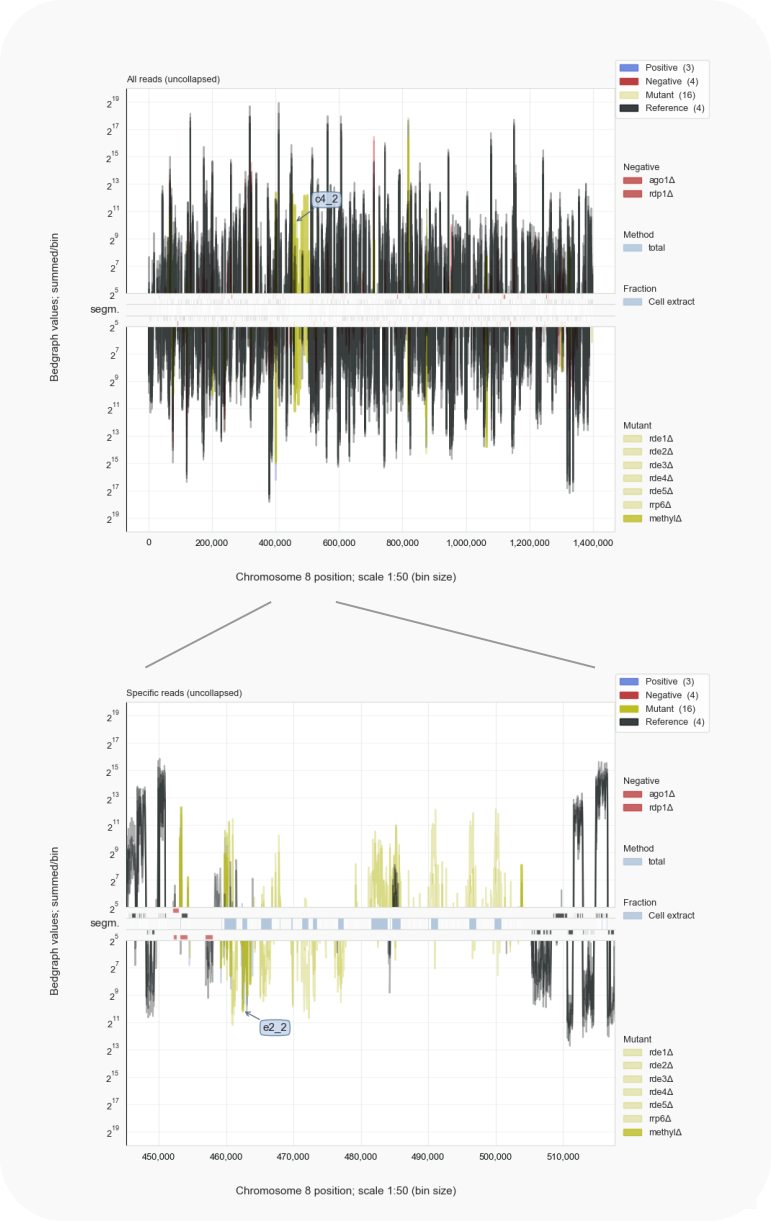
Figure 3. Visualization of centromeres.¶
Normally, centromeres are not transcribed, as indicated by the gap in mRNA transcripts (black). Inactivation of histone H3 methylation by deletions in genes for Clr4 (c4) or Ezh2 (e2) leads to loss of silencing of centromeric transcripts to which siRNAs are raised [Burke-2019]. With: coalispr showgraphs -c 8 -w2.
Graphs¶
Check traces:
coalispr showgraphs -c 8 -w2
Proteins involved in methylation of DNA and of histone H3, leading to heterochromatin formation and thereby silencing of genes in these chromosome regions, are important to reduce the production of siRNAs complementary to transcripts expressed from centromeres [Burke-2019]. Centromeres in Cryptococcus are long and consist of repetitive DNA derived from transposable elements [1]. The length of centromeres, together with the absence of RNAi against transposons in an organism, appears related to virulent behaviour of fungi [Yadav-2018]. The results from [Burke-2019] are helping to light up centromeric regions in bedgraph displays by Coalispr.
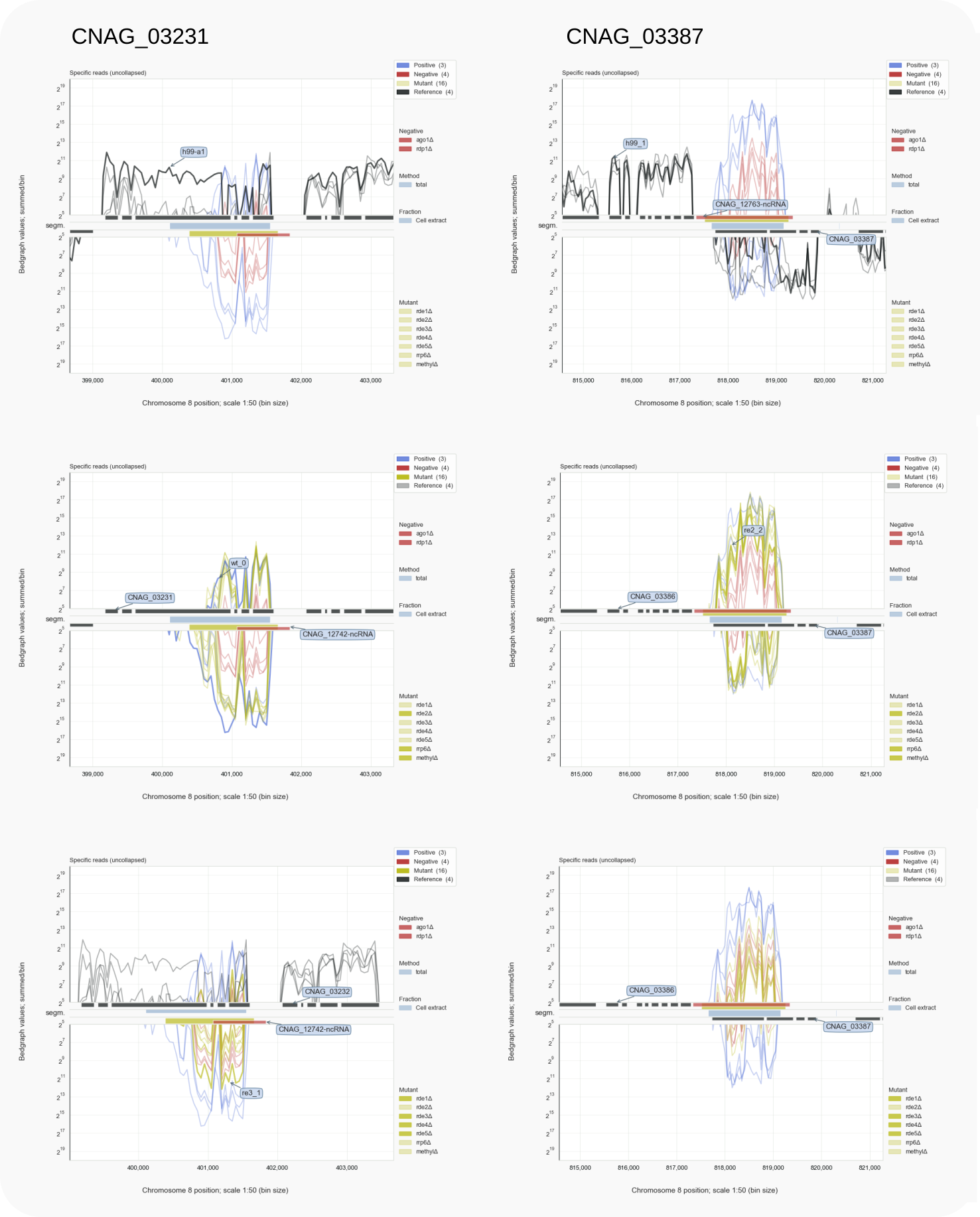
Figure 4. CNAG_03231 and CNAG_03387¶
Segments representing an siRNA locus have been annotated as a ncRNA. Not all mutants have an equal effect on siRNA formation. One group appears as wild type (middle) and another as the negative controls (bottom). Traces for Specific reads are shown (with: coalispr showgraphs -c8 -w4).
To revisit CNAG_03231, and see the impact of mutants on siRNA formation (see Fig. 4, run:
coalispr showgraphs -c8 -w4
And zoom in to regions 399000-403600 for CNAG_03387 or 814700-821200 for CNAG_03387. Alternatively, using the region option -r, for CNAG_03387 run:
coalispr showgraphs -c8 -w4 -r 399000-403600
For CNAG_03387:
coalispr showgraphs -c8 -w4 -r 814700-821200
This will show traces after filtering out the unspecific reads [15]. The C-terminal encoding regions of the transcripts are targeted by siRNAs. That introns have been removed by splicing is better visible in a genome browser; the 50-fold reduced resolution in the Coalispr display can only hint at this. Note that in absence of RNAi (in the h99-a1 mutant lacking Ago1) relatively higher mRNA expression levels are detectable for these loci compared to wild type (h99) and surrounding loci.
In the other tutorials, to support the article , rDNA loci have been analyzed.
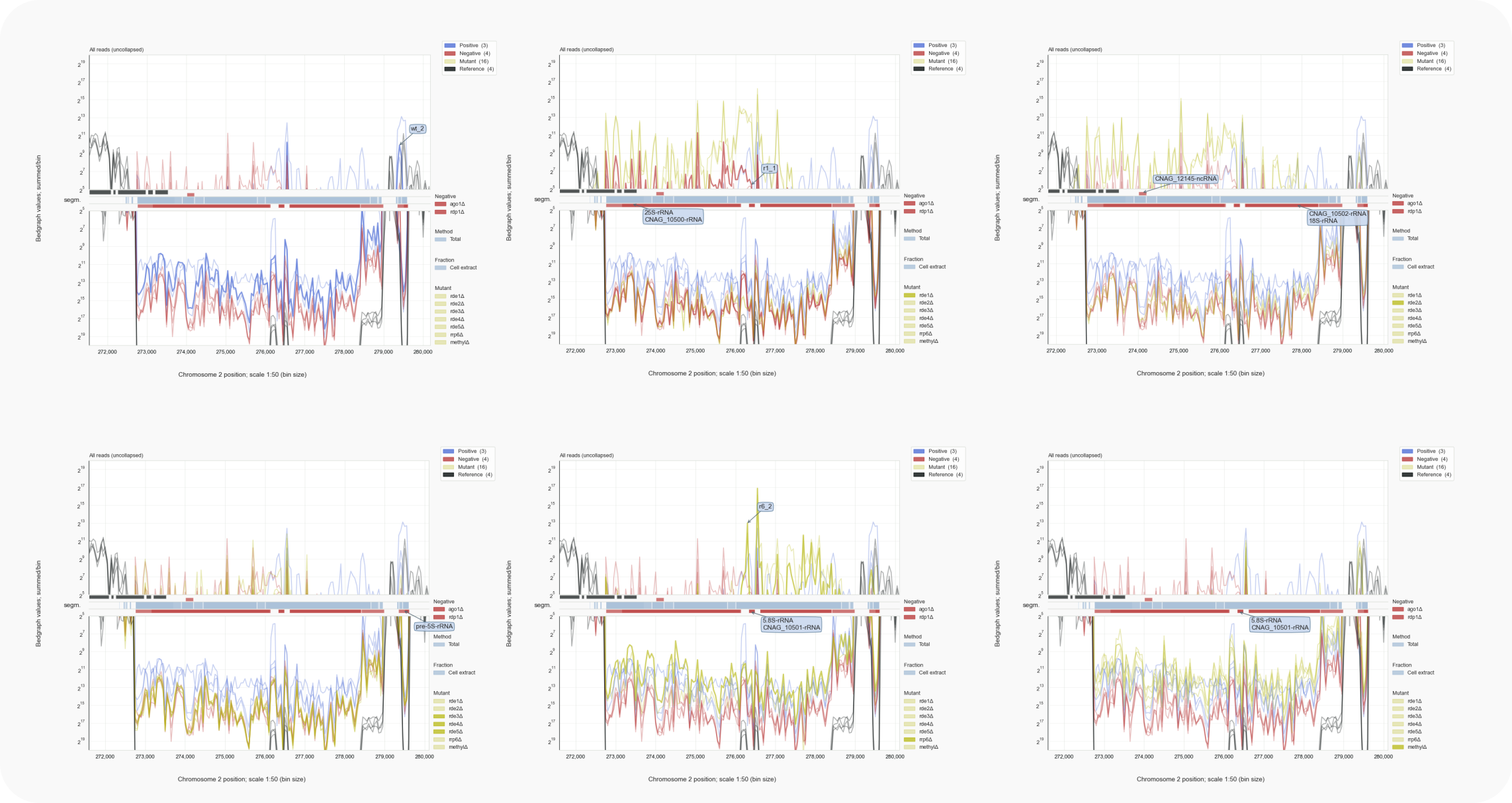
Figure 5. C. neoformans rDNA locus [16]¶
Reads representing rRFs are abundant in all samples to a comparable extent. In some clones, but not all, reads complementary to rRNA are formed. For the rde1Δ (top mid) and rde2Δ (top right) strains these reads counter regions of large subunit rRNAs 25S and 5.8S, while in wild type (top left) or rrp6Δ (bottom mid) the small subunit 18S rRNA region and flanking transcribed spacers are targeted. No effect is visible for strains lacking Rde3, Rde4 or Rde5 (bottom left) or in strains without DNA or histone methylation activity (bottom right). Although a contamination with rDNA might have caused this, the signals are typical for the strains and could have another source of origin. Note the rRNA peaks of the reference RNA-Seq libraries that run off the scale (black). Traces for all reads are shown (with: coalispr showgraphs -c2 -w2 ).
In Cryptococcus the rDNA is on chromosome 2, on the minus strand. All reads are shown with:
coalispr showgraphs -c2 -w2Using the
zoomtool, exposes the rDNA region from270000to284000
This command produces a display of traces that shows that rRFs are common to all samples. In some strains, including the wild type (from [Dumesic-2013]), reads are mapped that are antisense to the rRNA. Can these be the result of an RNAi response? Or are they generated by another, possibly artefactual mechanism?
Add count data¶
To obtain a more detailed insight in the kind of reads in the various libraries, we start with getting count data.
Counts for the input:
coalispr countbams -rc 1(for uncollapsed data)coalispr countbams -rc 2(for collapsed data)
Counts for aligned reads:
coalispr countbams(for specific data)coalispr countbams -k2 -u 1
With this information, we can inspect cDNA traces (of collapsed reads) with siRNA-like lengths and start-nucleotide [17] that are present in the negative data:
coalispr showgraphs -c2 -t2 -u1
This shows that such common small RNAs can derive from highly expressed transcripts (top left panel in the figure, including rRNA. Fitting the finding that siRNA populations consist of sense and antisense reads, such small RNAs are found to map to the rDNA, although mostly in a similar manner as found for the negative control lacking Rrp1 (r1). A fraction of reads that does not overlap with negative control reads is found in the wild type and a strain with mutant RRP6. Does this mean that rRFs are actually part of the siRNA population associated with Ago1 in a biologically relevant manner? Or is it that rRNA, rather than supplier, becomes a target of RNAi? Because of the mix-up with rRNA as common contaminant and the possibility that unselected siRNAs obtained their features by chance, these questions cannot be answered on the basis of these observations. For example, there is no idea about the length-distributions of the mapped reads; do the siRNA-like reads stand out from the remainder? This question is answered below.
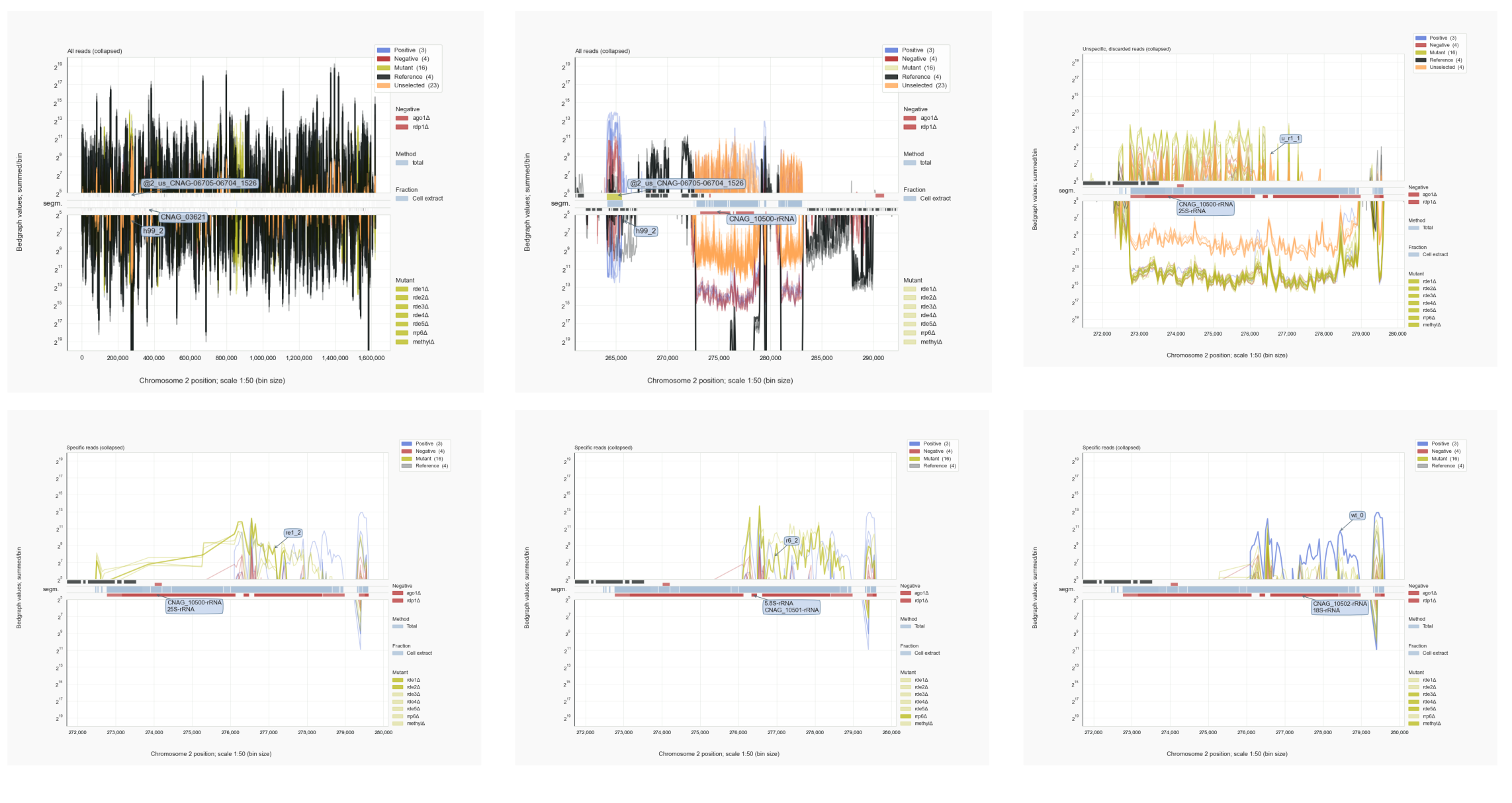
Figure 6. C. neoformans rDNA locus [16], mapped cDNAs.¶
cDNAs of unselected reads adhere to siRNA characteristics but fall in the class of unspecific sequences due to an overlap with common signals found in negative control samples. Many of these reads derive from highly expressed transcripts (orange traces on top of black mRNA signals, top left). This is also the case for the DNA regions with genes for rRNA (top middle), although a diverse population of cDNAs is found to be anti-sense to 25S and 5.8S or 18S rRNAs, which is also observed for the negative control lacking a functional RRP1 gene (top right). Anti-sense cDNAs against the 18S rRNA region and flanking transcribed spacers are specific for the wild-type strain or seen in the absence of Rrp6 (bottom) (with: coalispr showgraphs -c2 -t2 -u1 ).
Count diagrams¶
Coalispr is a tool to check libraries in a data set, and obtain counts for specified reads as done above. How different are the libraries with respect to coverage and number of mapped reads? For this, we can look at the uncollapsed and collapsed reads, respectively, using alignment information:
coalispr showcounts -rc 1coalispr showcounts -rc 2
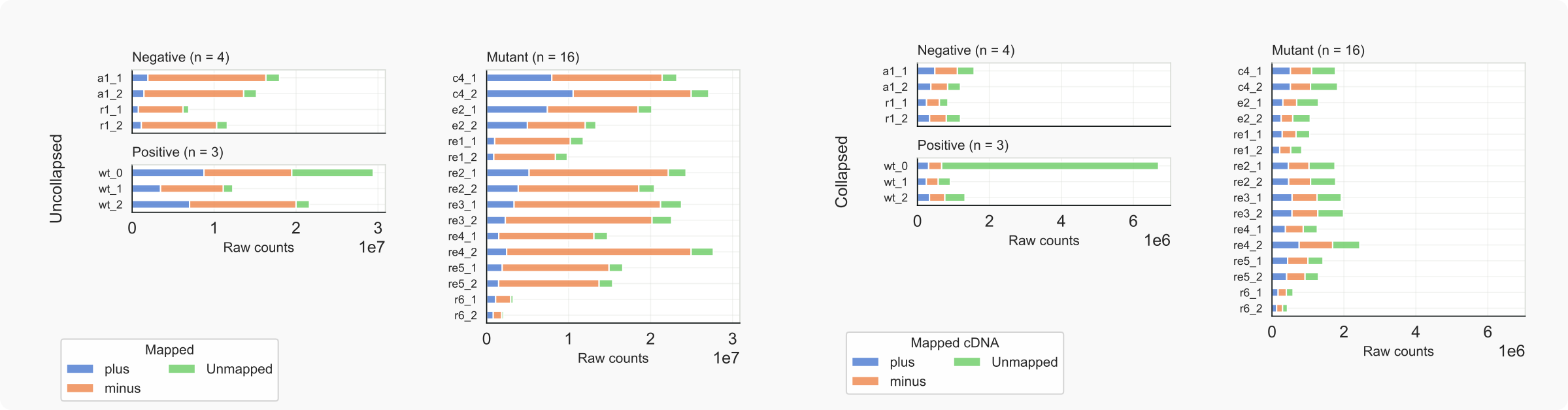
Figure 7. C. neoformans libraries, mapped (stranded) vs. unmapped reads.¶
According to these diagrams, the libraries are fairly comparable although the original data (wt_0) [Dumesic-2013] contain a high number of unmapped reads, possibly due to adapter remnants that interfere with the stringent EndToEnd mapping [9]. In general the background is fairly high. This observation is based on the overlap between the positive and negative control samples, with respect to a) the large fraction of common minus-strand reads (the rDNA gene is on that strand on chromosome 2) in the positive and mutant samples, while b) negative control samples contain reads that are specified as specific [18]. The characteristics for the libraries of mutant rde1Δ (re1) are very similar to that of the negative controls, suggesting that RNAi has been inactivated in the absence of Rde1. The strains lacking Rrp6 (r6) yielded a relatively low number of reads. According to Burke et al [Burke-2019] for all samples 20 μg RNA was used for library preparation of siRNAs. Assuming that experimental parameters for library preparation were comparable, are the low siRNA yields for rrp6Δ strains indicative for a poor state of these cells (slow growth, reduced RNA synthesis or minimal RNAi activity)?
Checking the overall counts for specific and unspecific reads gives other information:
coalispr showcounts -lc {1-7}
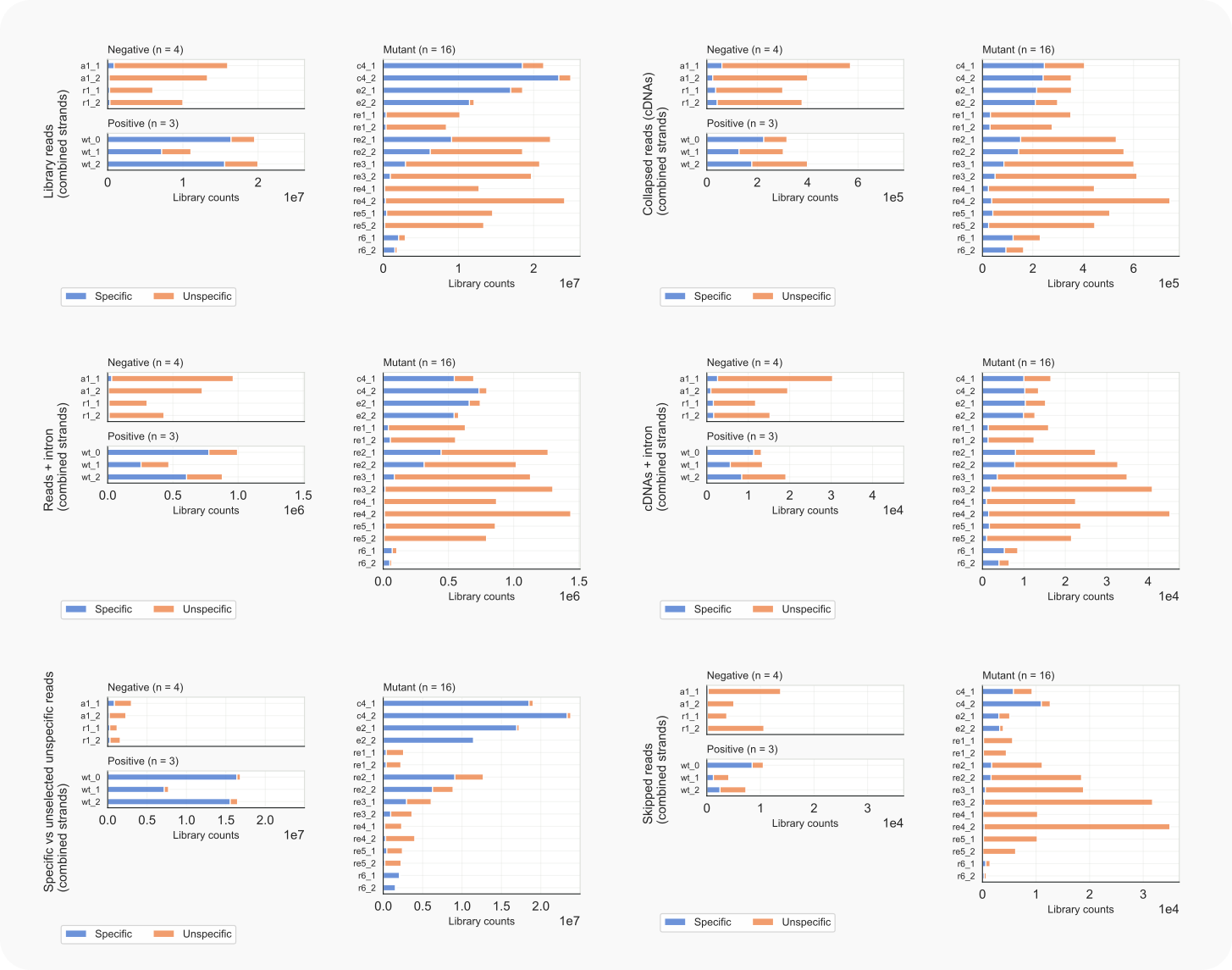
Figure 8. C. neoformans library counts, specific vs. unspecific reads.¶
-lc 1; left) and collapsed data, cDNAs (-lc 2; right).-lc 4; left), and in cDNAs (-lc 5; right).-lc 6; left); reads skipped during counting (-lc 7; right).The results obtained with option -lc 1 indicate that RNAi activity seems almost completely abolished not only in mutant rde1Δ, but also in rde4Δ, rde5Δ and possibly rde3Δ (re1, re4, re5, and re3, resp.). This can be inferred from the finding that (nearly) all mapped reads are part of a common background (top left). In mutant rde2Δ (re2) RNAi appears to be affected as well, although partially, in view of the enhanced number of unspecific reads at the expense of those mapping to specific siRNA targets. Note that an opposite observation, more specific and less unspecific reads, can be made for mutant clr4Δ (c4)
The fact that Cryptococcus is intron-rich is exemplified by the many specific reads with gaps that could be introns (about one in 25 cDNAs; middle).
One to three percent of mapped reads were skipped during the counting because their end-to-end alignment was not solely based on matches with, possibly, one intron-sized gap (i.e. larger than MININTRON; cf. bottom right to top-left).
Length distributions of the mapped reads are visualized with:
coalispr showcounts -lo {1-5}for an overview for all libraries, and, for individual samples:coalispr showcounts -ld {1-6}with option-k2lengths for unspecific reads are shown and with option-malengths for uniq reads or multimappers (see below).
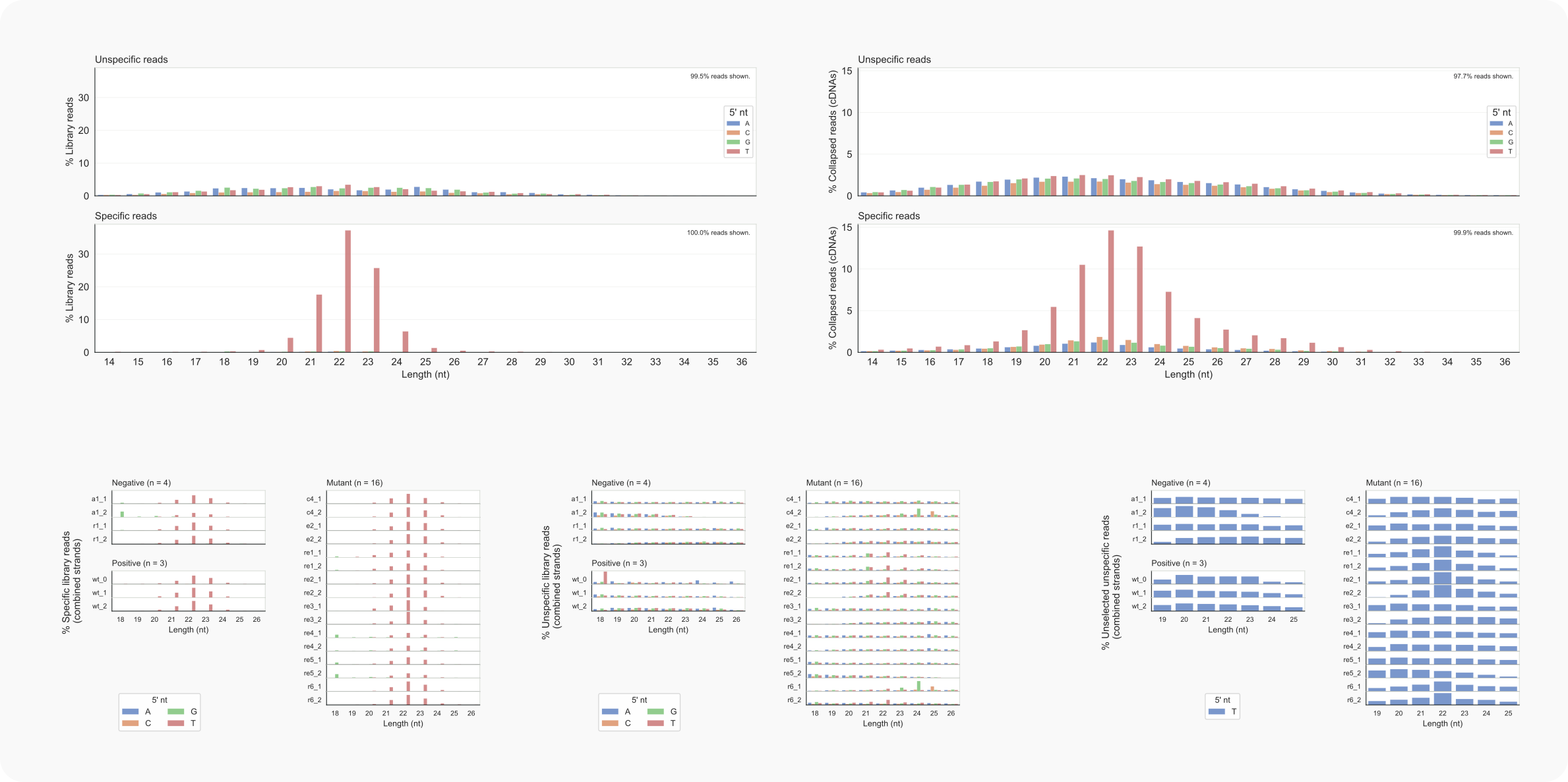
Figure 9. C. neoformans length distributions of library reads.¶
-lo 1) and collapsed data (cDNAs; -lo 2) on the right.-ld 1, left), unspecific (-ld 1 -k2, middle) and unspecified reads in unspecific data (-ld 6; right).The distribution of read lengths to a narrow range of 20-24 nt with the majority starting with a T (U in RNA) fits expectations for siRNAs bound to Argonaute proteins in fungi [Dumesic-2013]. Furthermore, the separation of specific reads from unspecific reads seems to be adequate in view of the hugely different length distributions for these categories of reads. That putative siRNAs are still present among the unspecific reads in the rde1Δ, rde2Δ or rrp6Δ strains (re1, re2, or r6, bottom middle) appears to be supported by length-distributions for these unselected reads (bottom right) and observations for the rDNA region described below (see Region-analysis).
Multimappers¶
Transposons that elicit an RNAi response are often present in multiple copies, although some loci might be more active than others, possibly depending on an accumulation of mutations. Therefore, it is not obvious how to account for such repeats during mapping. In the alignment scripts, STAR was set up to map uncollapsed multimappers randomly to the possible loci, while collapsed reads are aligned with each possible position (to keep these hits visible in the bedgraphs) but counted as 1/NH (with NH the number of hits found in the bam alignment line).
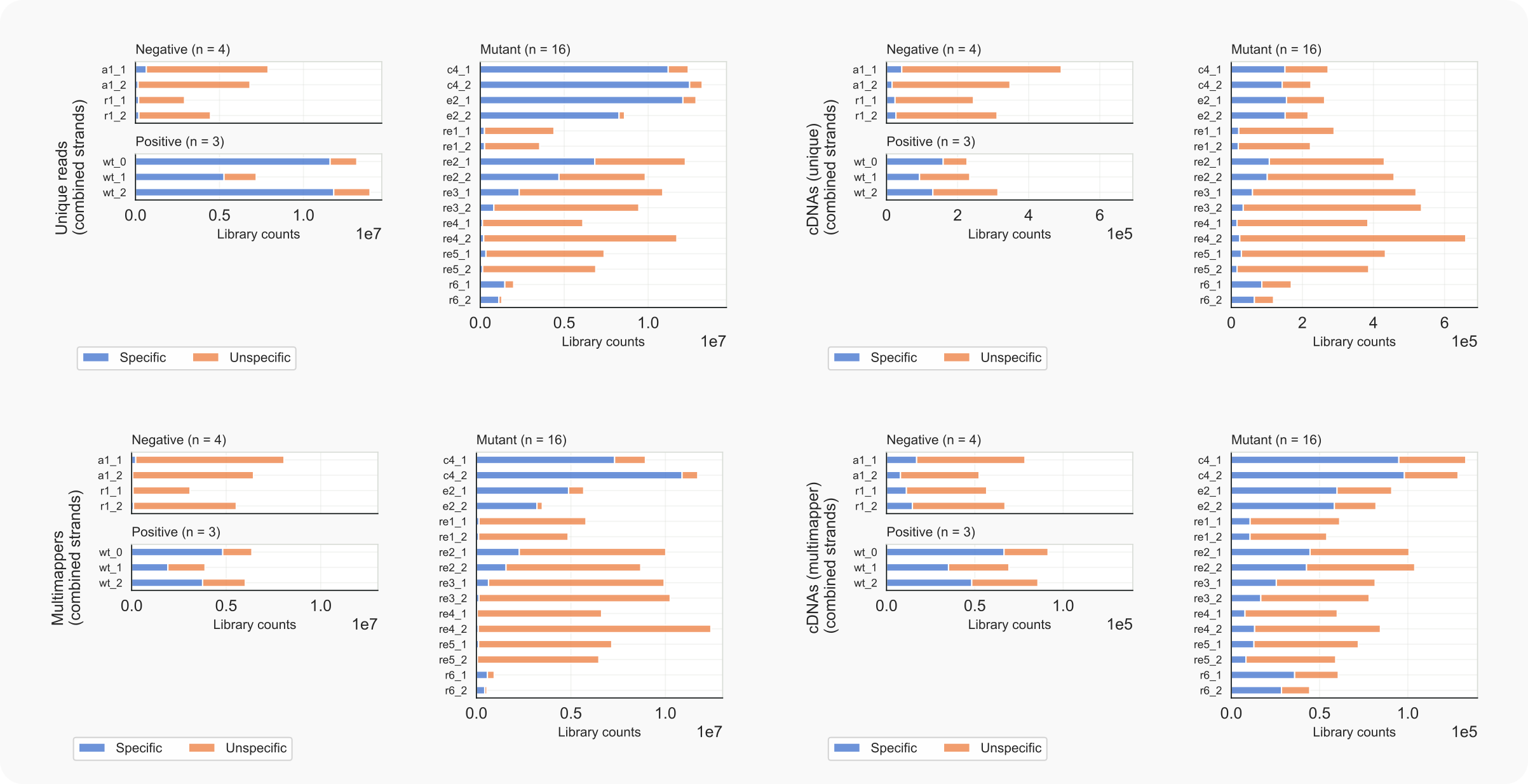
Figure 10. C. neoformans library counts, specific vs. unspecific reads, with focus on unique reads and multimappers.¶
-lc 1 -ma 1, left) or unique cDNAs (-lc 2 -ma 1, right).-lc 1 -ma 2, left) or multimapping cDNAs (-lc 2 -ma 2, right).The above figure indicates that most of the reads and cDNAs align to a single site (’unique reads’, top) in the reference genome rather than to multiple loci (‘multimappers’, bottom). Centromeres are normally silenced by packaging of nucleosomes into heterochromatin, which coincides with methylation of H3K9 (lysine 9 in histone H3) by Clr4. When this methyltransferase is absent (Mutants c4), the number of multimappers increases (relative to total and unique populations) compared to wild type (Positive wt). Centromeres in Cryptococcus consist mainly of repetitive sequences derived from retrotransposons Tcn1–Tcn5 (that belong to the Ty3-gypsy family of retroelements) and Tcn6 (like Ty1-copia) [Yadav-2018]. Therefore, the increase of multimappers in the absence of Clr4 fits a scenario whereby centromeric transcripts, the expression of which is no longer suppressed by methylation, are silenced by RNAi instead. Notably, genes on euchromatic regions of the chromosomes to which large numbers of siRNAs align to often share homology with domains found in transposable elements but are not clearly related to Tcn1-6.
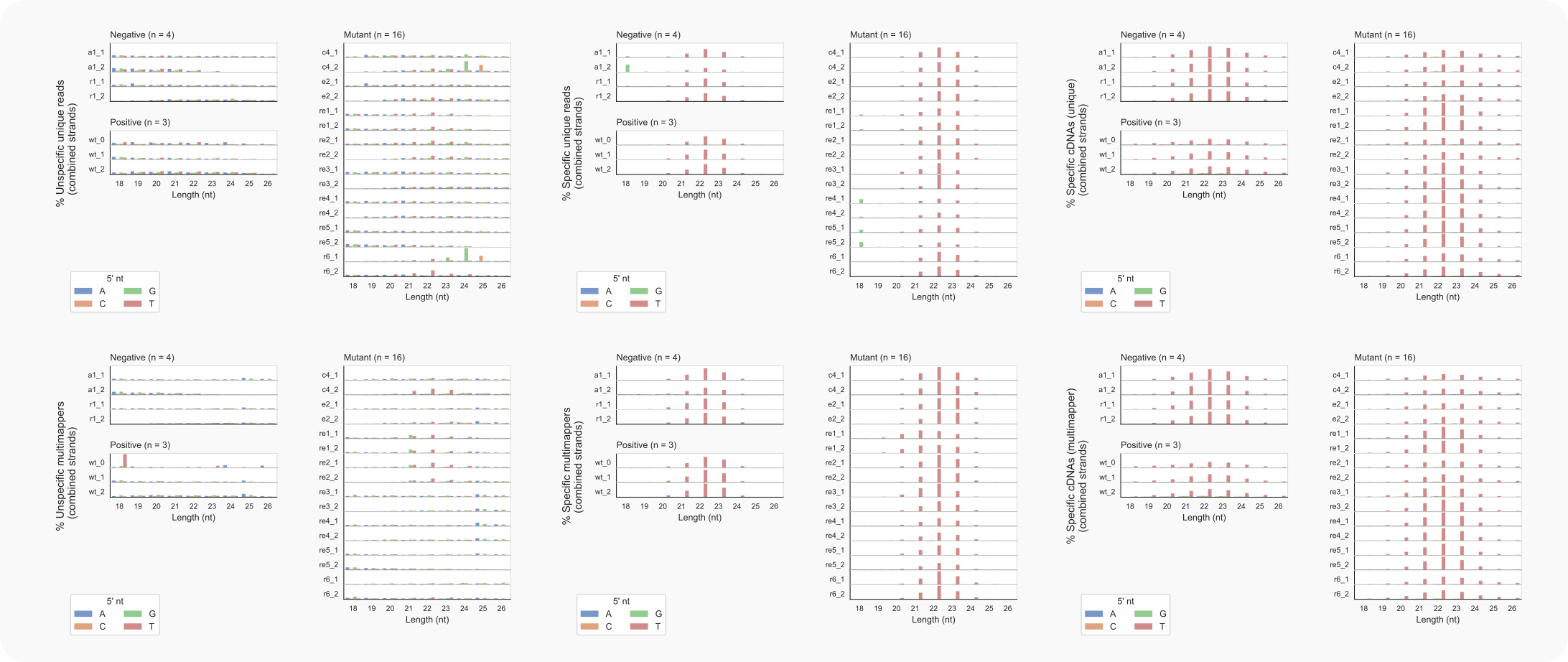
Figure 11. C. neoformans length distributions of unique siRNAs and multimappers.¶
-ma 1, top) and multimappers (-ma 2, bottom);In the absence of Rde1 only a few siRNAs are found (see Figure 8), defined by cDNAs with a wild-type length distribution (coalispr showcounts -ld 2 -ma {1,2}). Still, most of the multimapping siRNAs (coalispr showcounts -ld 1 -ma 2) in this mutant appear to be 1 nt shorter, peaking with a length of 21 nt, instead of 22 nt (Fig. 11, middle panel, bottom row). Section ‘Multimapping 21-mers in rde1Δ’ tries to find loci responsible for this. One candidate turns out to be the rDNA locus, where such siRNAs map against the pre-rRNA transcript.
Intron-skipping siRNAs¶
With options -lo {4,5} or -ld {4,5} intron lengths are displayed in uncollapsed reads or collapsed cDNAs, respectively [19]. For a large range of lengths no hits are present. The resolution of the distribution can be enhanced by skipping this empty section. For this:
Edit the field SKIPINT in
3_h99.txt, set it to= 190, 350and save the file.Activate the new configuration by rerunning the
setexp -e h99 -p2command.Redraw the figures.
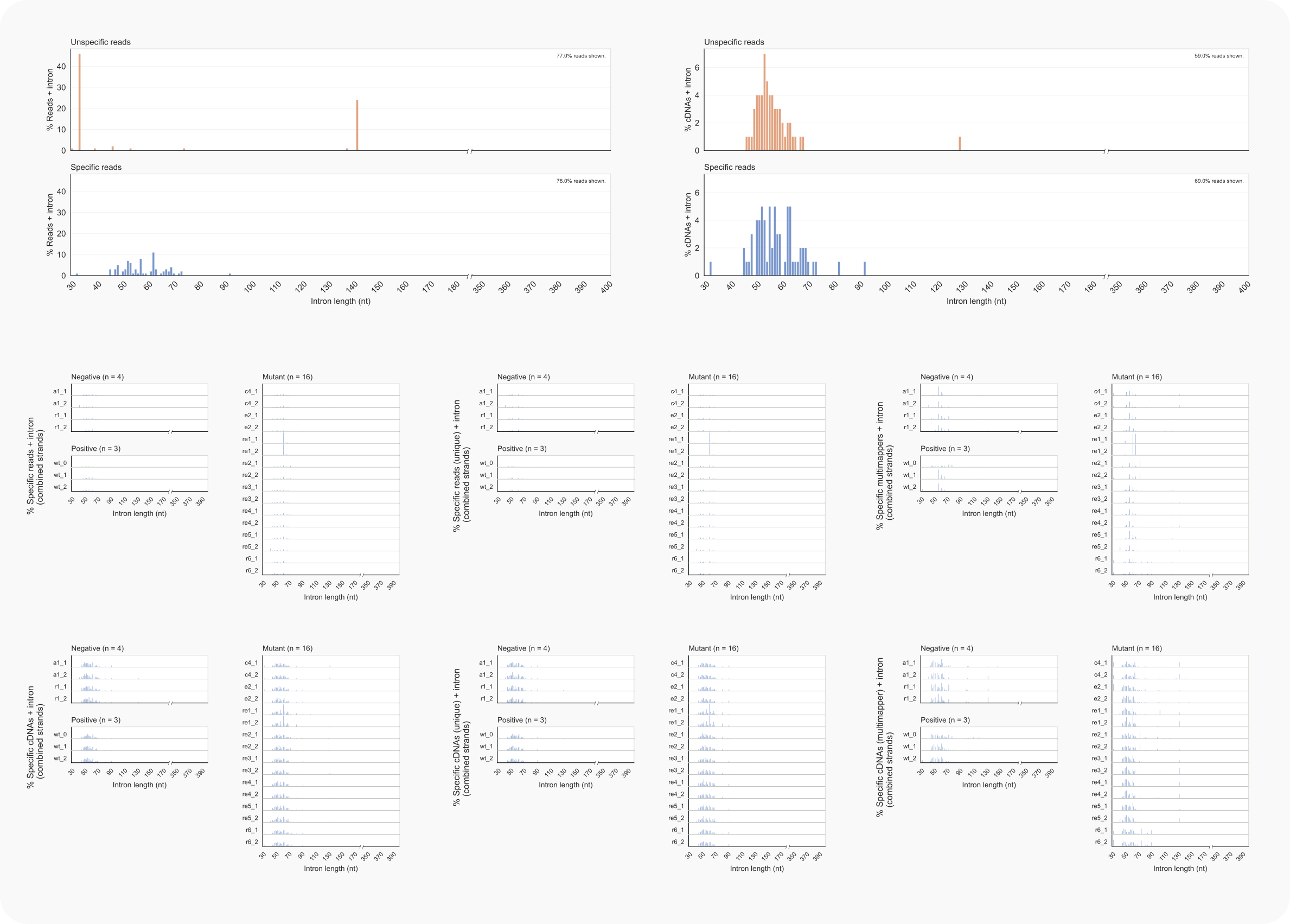
Figure 12. C. neoformans intron lengths of library reads.¶
-lo 4) and collapsed data (cDNAs; -lo 5) on the right.-ld 4) or cDNAs (Bottom, left -ld 5), unique (middle, -ld {4,5} -ma 1) or multimappers (right, -ld {4,5} -ma 2) for separate libraries.Of the intron-skipping reads, both unique and multimappers bridging an intron of ~62 nt are enriched in the absence of Rde1 (lanes re1 middle section); the number of cDNAs is also relatively increased for these reads, predominantly for those that align to one locus (bottom section, left and middle vs. right panels. In cells without Rde2, multimappers (reads as well as cDNAs) over a spliced intron of ~73 nt stand out (lanes re2 middle and bottom, right). Based on this information, the section ‘Intron-skipping siRNAs’ describes how putative loci can be identified for these reads.
The unique locus from which siRNAs with a 62 nt gap are accumulating in the absence of Rde1 turns out to harbour a unique pseudo-gene, CNAG_07650, on chromosome 6. Centromeric loci account for the multimapper in rde2Δ cells.
Region-analysis¶
Sometimes, read counts and length-distributions that are valid for just a small section of the overall transcriptome can be highly infomative. Coalispr facilitates this with the coalispr region call. For example, let’s analyze in more detail the rDNA unit in C. neoformans.
rDNA¶
Obtain images and coordinates for the complete rDNA in H99 from a graph displaying all reads:
Run
coalispr showgraphs -c2 -w2.Use the zoom-tool to select the region covered by the large peak near position 300.000 [16].
Activate mutant traces with comparable traces (
rde1Δ, rde2Δ,rde3Δ, rde4Δ, rde5Δ, methylΔ, orrrp6Δ) and save each image as png.Clicking the x-axis will generate in the terminal the genomic coordinates for the region displayed; e.g.
2 (271985, 279663).Highlight the coordinates (
271985, 279663) and copy these withShift+Ctrl+Cto the clip-board.
Get counts for the rDNA and count-displays by:
In another terminal type ``coalispr region -c 2 -r ``
After option
-r ``, paste the coordinates copied above with ``Shift+Ctrl+V.To use all available samples, include option
-s 2.Set up strand-specific analysis by including option
-st.Run
coalispr region -c2 -r 271985, 279663 -s2 -st 2for PLUS reads and associated diagrams.
The terminal will display which samples are counted; the number of skipped reads, a dataframe showing total numbers and feedback for the figures that are made and automatically saved to the indicated folders [21]. The generated count data are saved as separate files in
/<path_to/Burke-2019/Coalispr/data/h99/tsvfiles/region_readcounts_collapsed-bam/region_2_271985-279663[22].Run
coalispr region -c2 -r 271985, 279663 -s2 -st 3for MINUS reads and associated diagrams.
The obtained diagrams have been combined into Figure 13:
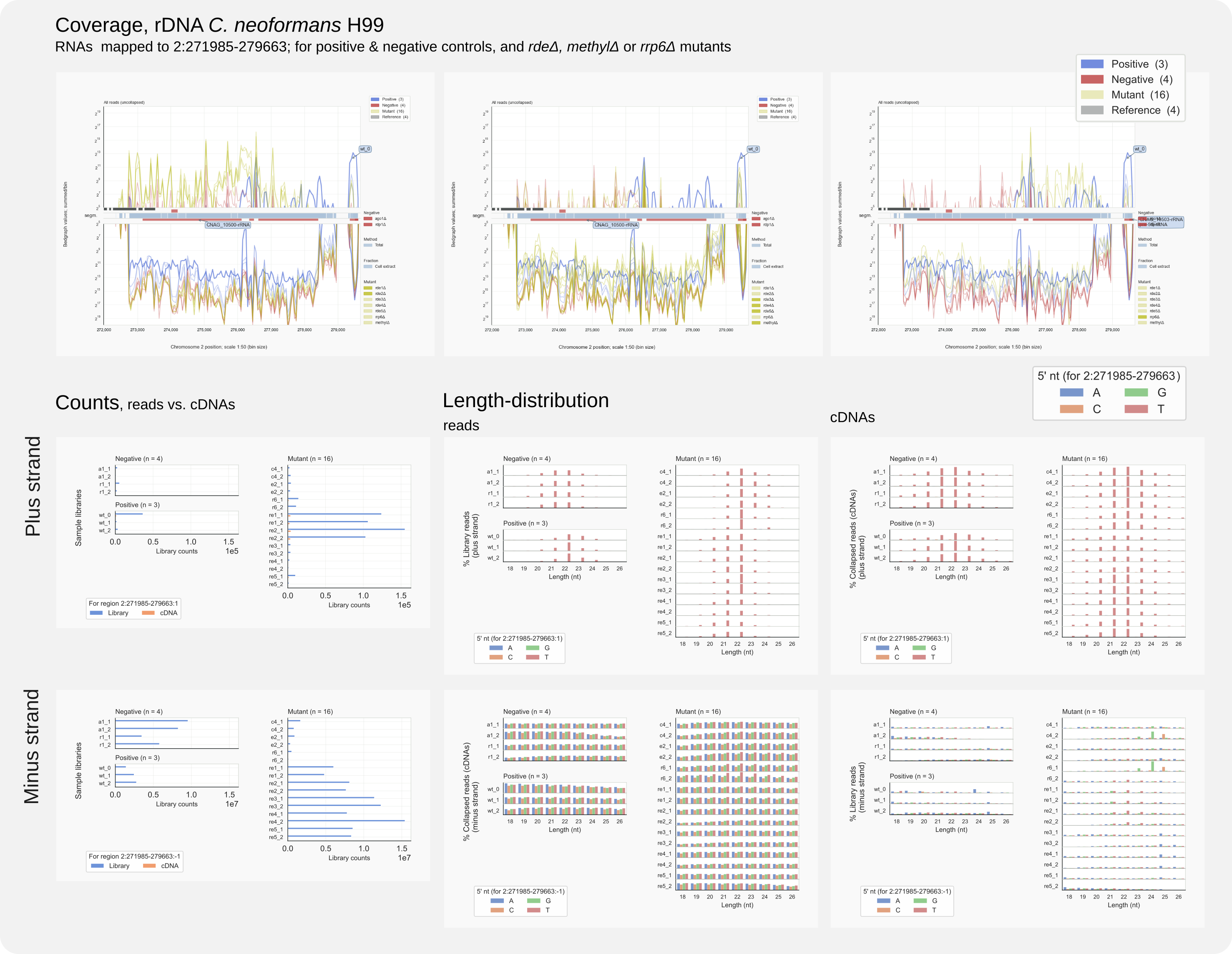
Figure 13. Region analysis of C. neoformans rDNA on chromosome 2.¶
Various bits of information can be distilled from this figure. When focusing on reads mapping to the rDNA sense strand (minus strand):
RNA fragments formed from ribosomal RNA (the rRFs) are very abundant in negative controls and rde mutants affecting RNAi (
re1tore5) and more so than in strains producing siRNAs (Positive) that could participate in reverse transcription reactions that create the input for the sequencing (see bottom row, left panel).rRFs form the majority of overall unspecific library counts (cf. Fig. 8, top left panel)
The rRFs do not meet the criteria for Ago-associated small RNAs that have a length of 19-23 nt and start with a U (T in cDNA) (see bottom row, middle panel for reads and right panel for cDNAs [23]).
That, despite the high number of counts, rRFS represent uninformative background is illustrated by the coverage pattern of these RNAs, which is the same for all samples (see top panel).
Interestingly, RNAs mapping to the antisense rDNA strand, suggest that siRNAs have been generated against rRNA:
A large number of antisense, plus strand reads were counted for strains rde1Δ (
re1), rde2Δ (re2) and a positive control (wt_0) (Middle row, left panel). See also Fig. 16 (top) below.In all samples, these antisense reads conform to siRNA characteristics.
Different from wild type siRNAs, those in mutant strains appear in general to be 1 nt shorter.
It cannot be ruled out that for samples with low counts these putative siRNAs are an experimental artefact or a kind of background. Still, the high levels of these reads for rde1Δ and rde2Δ strains suggest that in these mutants the RNAi activity could have been redirected to rRNA from transcripts that, somehow, no longer form a target. Thus, a step that would have lead to the generation of specific siRNAs against transcripts from pseudo-genes or transposable-elements is no longer happening because of these mutations.
21-mers¶
Are the siRNAs only shorter for the rDNA locus in strains rde1Δ or rde2Δ? We can check this for CNAG_07650, one of the few loci with siRNAs in the absence of Rde1 (see Figure 12). The coverage graphs in the section ‘Intron-skipping siRNAs’ show that the major target is the transcript from this pseudo-gene on the plus strand. After zooming the display to only show the region with siRNA traces, obtain the coordinates (say 1371800-1372650) by clicking the x-axis and copy-paste the numbers to run:
Run
coalispr region -c6 -r 1371800-1372650 -s2 -st {2,3}
The combined output indicates that at this locus siRNAs are not shorther in mutant cells, compared to wild type:
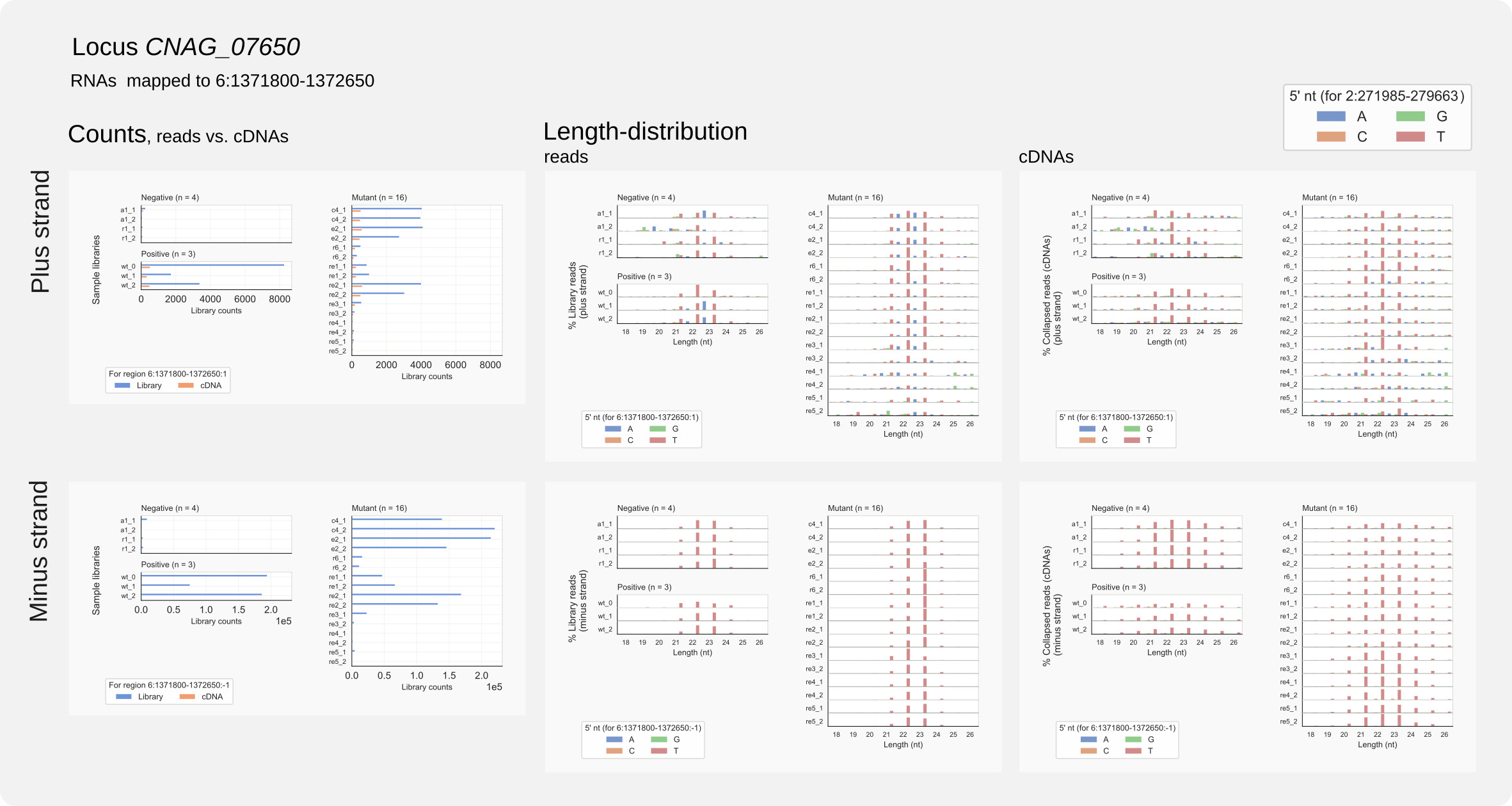
Figure 14. Region analysis of C. neoformans CNAG_07650 on chromosome 6.¶
CNAG_07650 sequences are not repeated in the H99 genome and this outcome fits the length-distribution for unique loci (Fig. 11, middle panel, top row).
For rde1Δ cells a multimapper was responsible for relative accumulation of siRNAs of 21 nt (see Fig. 11 middle panel, bottom row). This quick analysis suggest that shortening of siRNAs could be linked to the rDNA locus.
Note that for strains with inactivated RNAi (ago1Δ or rdp1Δ), RNAs are still mapping to the CNAG_07650 locus with siRNA length-distributions. For other RNAi loci such background is observed as well. Whether incomplete RNAi-inactivation or library contamination could be the reason for this, is unclear.
auto-RNAi¶
Above analysis for the rDNA showed that in some strains siRNAs are detected that are antisense to rRFs (Fig. 13). Are other common ncRNAs prone to be targeted by RNAi? It looks that this can happen in the case of SRP RNA as well, another prominent source of unspecific reads. SRP RNA gets expressed from the minus strand on chromosome 1 and is part of a cytoplasmic RNP that associates with ribosomes to regulate translation of proteins that localize to the ER.
Run
coalispr showgraphs -c1 -r 801150-801450 -w2for the coverage display.Run
coalispr region -c1 -r 801150-801450 -s2 -st {2,3}for the count diagrams.
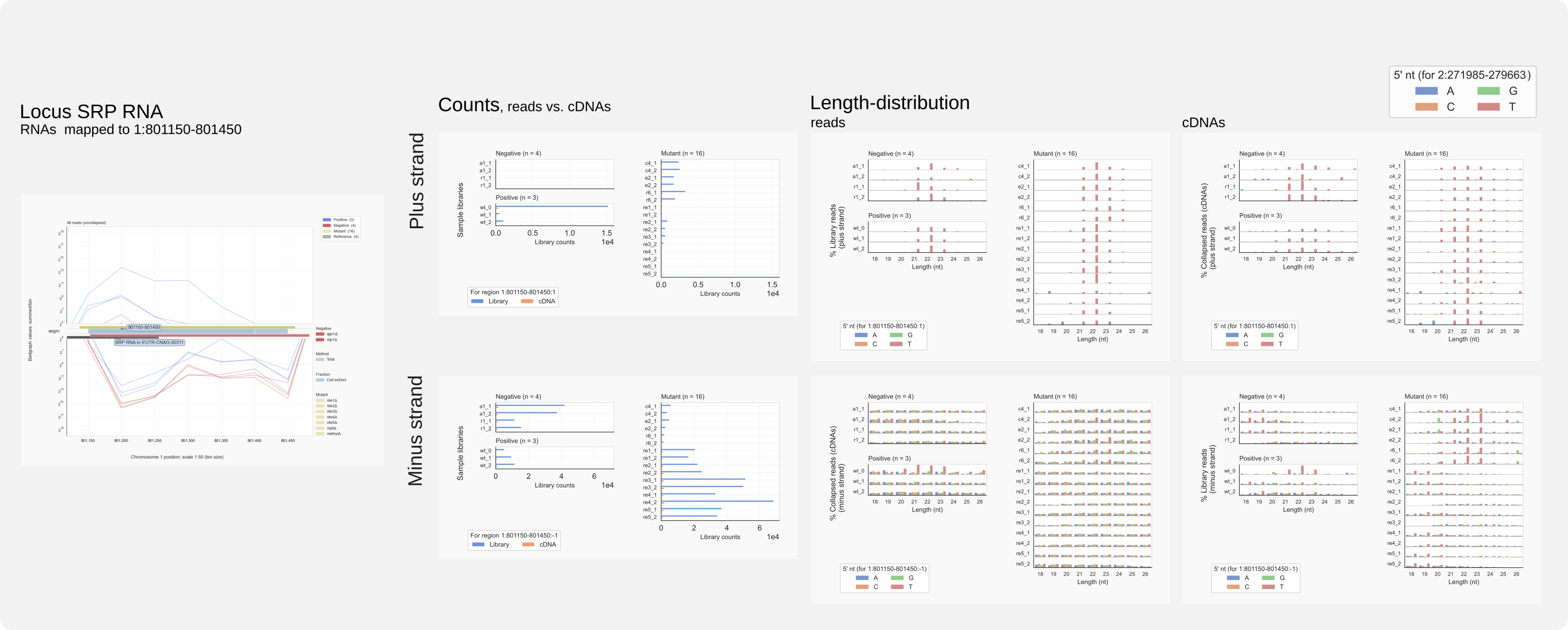
Figure 15. Region analysis of C. neoformans SRP RNA locus.¶
Note that, for this unspecific locus in RNAi capable strains, cDNAs are found representing CORB sense siRNAs and the more abundant MUNR antisense siRNAs. In strains where RNAi has been affected, the cDNAs for CORB cDNAs do not adhere to a siRNA length-distribution. At loci with specific reads, such reads were observed in low amounts for strains where RNAi had been inactivated (for example CNAG_07650). Such ‘background’(?) signals may have been too low at unspecific loci to stand out as siRNAs in these strains.
If these siRNAs against ncRNAs are really formed, these siRNAs show activity against molecules representing ‘self’, like a sort of auto-immune response but then for RNAi, ‘auto-RNAi’. This also occurs in C. deneoformans, against similar ncRNAs (rRNA, SRP RNA). Such events might indicate that processes involving these ncRNAs triggered RNAi. A common factor is translation. [26]
Annotate count files¶
The available reference files with genome annotations can be scanned for overlap with segments to which siRNAs map. For this run the coalispr annotate command. Include the mRNA references with -rf 1 an, to easier assess large count numbers, take their log2 values:
coalispr annotate -lg 1 -rf 1
And for unspecific reads (-k 2):
coalispr annotate -lg 1 -rf 1 -k2
To get counts for unspecific reads opposite rRNA sequences, include the CORB strand option (-st 3):
coalispr annotate -lg 1 -rf 1 -k2 -st 3
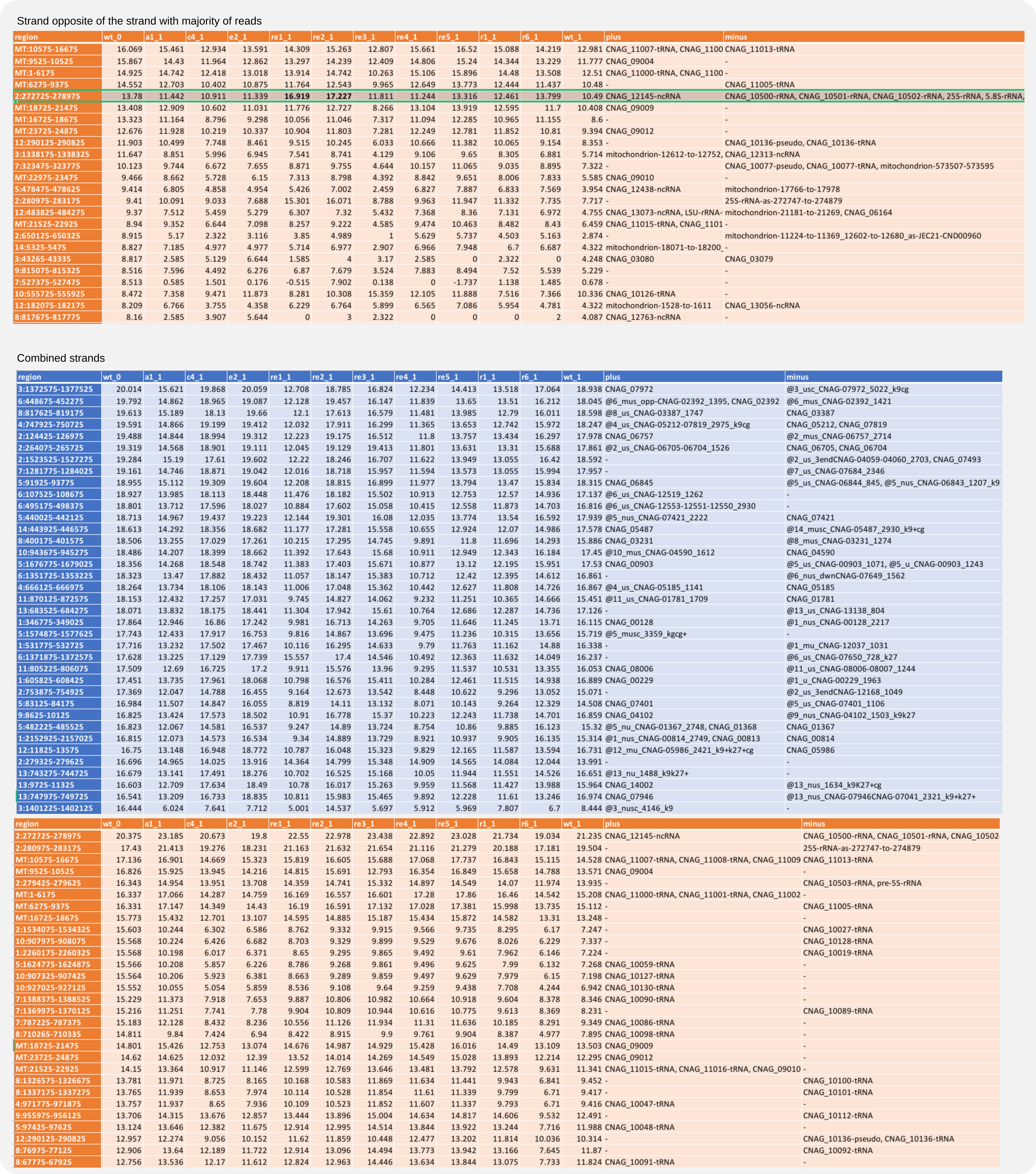
Figure 16. Annotated counts (log2 values).¶
As found for mouse, in these small RNA-Seq libraries for C. neoformans the counts for rRFs are among the highest read numbers. Most unspecific reads relate to rRNA, tRNA and mitochondrial transcripts; most specific reads are opposite uncharacterized genes.
Conclusion¶
In the analysis of siRNAs and unspecific RNAs for various libraries prepared by [Dumesic-2013] and [Burke-2019] many observations were linked to rde1Δ and rde2Δ. RDE1 (CNAG_01848) codes for a G-patch protein similar to Pfa1/Sqs1 in Saccharomyces cerevisiae, one of the cofactors for Prp43. This multi-functional RNA helicase relies on G-patch proteins for functional specificity [Bohnsack-2022]. In yeast, the Prp43/Pfa1 complex is linked to the last step in the maturation of 18S rRNA, cytoplasmic cleavage of 20S pre-rRNA [Lebaron-2009, Pertschy-2009]. In association with different G-patch proteins, Prp43 is further involved in dissociation of spliceosomes and the removal of snoRNPs responsible for 2’-O methylation of 18S and 25S rRNA [Bohnsack-2009].
Other RNA-processing factors were identified by Burke et al., namely the RNAse III Rnt1 (CNAG_06643, or RDE3), which, in yeast, is a nuclear protein (for a review see [Elela-2018]) and involved in co-transcriptional pre-rRNA processing, analogous to bacterial RNAse III [Abou Elela-1996], and maturation of snoRNAs such as U14 [Chanfreau-1998a, Chanfreau-1998b]. Overall, 13% of the yeast genes are differentially overexpressed in rnt1Δ cells. Yeast Rnt1 carries a characteristic C-terminal RNA-binding domain (RBM0) not present in homologous proteins in fungi that harbour an RNAi machinery. Cryptococcus Rnt1/Rde3 lacks this domain [25] and therefore might not be constrained to double strand RNA substrates formed by NGNN stemloops recognized by RBM0 as is the case for yeast Rnt1 [Elela-2018]. Rde5 (CNAG_04791), with no known orthologues, appeared to be a Rnt1-interacting protein [Burke-2019]. Rde2 (CNAG_04954) is placed among the orthologues of human EDC4, an enhancer of mRNA decapping enzymes, while Rde4 (CNAG_01157) contains domains that suggest it is an orthologue of the TENT5 family of terminal nucleotidyltransferases involved in polyadenylation during 3’ end processing of RNA and linked to the regulation of the innate immune response in animals [Warkocki-2018, Liudkovska-2022].
Figure 17. RNAi onset occurs after splicing.¶
Disruption of these factors activated the Harbinger transposon to jump from the context of a URA5 reporter gene, and resulted in reduced siRNA production. Because such deletions can be expected to primarily affect ribosome synthesis or mRNA degradation, it cannot be ruled out that a subdued RNAi response could be an indirect result of these deletions. Information from experiments that assess splicing, RNA turnover, and maturation of pre-rRNA and ribosomal subunits in rdeΔ strains will be needed to understand what is actually happening in these cells.
Either way, in rdeΔ strains an RNAi response would be alleviated when this process is triggered by stalled ribosomes and thus directed to ‘difficult’ mRNAs that cause this (Fig. 17). After ribosomes have stalled, a quality control mechanism involving mRNA degradation is initiated by decapping enzymes and 3’ end processing. A lack of sufficient numbers of ribosomes might reduce their chance of collapsing into each other and thereby the onset of clearing processes that also involve RNAi and the generation of siRNAs.
The model cannot explain how siRNAs are maintained and propagated during cell division if the relevant transcripts are not synthesized [24]. That translation might provide a relevant context for the onset of RNAi in Cryptococcus is indicated by the fact that many siRNAs mapped to pseudo-genes and TE-loci, but not - in C. deneoformans - to T1 [Janbon-2010], an active, non-coding transposable element [van.Nues-202?]. Recently it has been found that in stem cells of planarians (a kind of flatworm) piRNA generation is associated with the pioneer round of translation, directly involving the argonaute-like PIWI protein SMEDWI-1 [Parambil-2023]. Thus, route 1 in the model, priming RNAi on problematic transcripts, might occur during such a pioneer round or through common translation on cytoplasmic ribosomes [27]. Another hint in the case of Cryptococcus might be given by the amounts of siRNAs that map anti-sense to mature rRNA (Figure 13), or SRP RNA (Figure 15), in rde1Δ strains and in repair strains where RNAi has been re-established by restoration of genes for Ago1 or Rdp1 [van.Nues-202?]. Maybe such RNAs are mistakenly turned into targets when stalled translation complexes are resolved. Would this also mean that cells need to “learn” that some RNAs, like rRNA, are self?
Notes¶
Trace for the run that generated count panels for the MINUS strand of the figure:
bash-5.2$ coalispr region -c2 -r 271985, 279663 -st 3 -s2
a1_1
Skipped: 7818
a1_2
Skipped: 2810
r1_1
Skipped: 2145
r1_2
Skipped: 6073
wt_0
Skipped: 850
wt_1
Skipped: 1696
wt_2
Skipped: 2714
c4_1
Skipped: 1932
c4_2
Skipped: 733
e2_1
Skipped: 1200
e2_2
Skipped: 420
re1_1
Skipped: 3099
re1_2
Skipped: 2328
re2_1
Skipped: 5418
re2_2
Skipped: 9850
re3_1
Skipped: 10652
re3_2
Skipped: 18727
re4_1
Skipped: 5889
re4_2
Skipped: 20248
re5_1
Skipped: 5768
re5_2
Skipped: 3520
r6_1
Skipped: 487
r6_2
Skipped: 196
Sample Library cDNA
a1_1 9567157.67 74940.15
a1_2 8280898.83 49650.90
r1_1 3500283.50 56307.24
r1_2 5818569.31 66155.82
wt_0 1429203.39 29205.80
wt_1 2483356.90 43138.73
wt_2 2793111.66 46670.00
c4_1 1685970.08 44513.99
c4_2 828864.22 31669.26
e2_1 923545.79 39107.84
e2_2 354180.50 30570.28
re1_1 6038546.63 56257.64
re1_2 4826315.55 51447.41
re2_1 8137399.37 63894.66
re2_2 7681477.36 76206.80
re3_1 11405806.14 70610.67
re3_2 12266882.70 82820.17
re4_1 7813346.08 62386.71
re4_2 15429512.61 86797.03
re5_1 8570380.14 66718.64
re5_2 8382610.76 56833.83
r6_1 526533.19 29702.34
r6_2 119248.39 18477.06
Showing counts of Library, cDNA reads for minus strand(s) of 2:271985-279663 23 samples, grouped by category.
Building figure...
Saving to file in /<path_to>/Burke-2019/Coalispr/figures/h99/svgfigures/regions
Figure finishing.
Showing library length distributions for minus strands of region 2:271985-279663 as bar graphs for 23 separate libraries, grouped by category.
Building figure...
Saving to file in /<path_to>/Burke-2019/Coalispr/figures/h99/svgfigures/lengthcounts
Figure finishing.
Showing cDNA length distributions for minus strands of region 2:271985-279663 as bar graphs for 23 separate libraries, grouped by category.
Building figure...
Saving to file in /<path_to>/Burke-2019/Coalispr/figures/h99/svgfigures/lengthcounts
Figure finishing.
The path is generated from constants (DATA, EXP, SAVETSV, REGI, READCOUNTS, and TAGBAM)
The length-distribution shows percentages of molecules with a given length in a sample. The cDNAs (or collapsed reads) will only show peaks if many molecules of the same length are formed; but peaks are low when many molecules of different lengths are in the sample. Molecules outwith the range given in the figure are not shown, but are contributing towards the denominator; so many are possibly longer than 26 nt.
When starting from RNAi-null strains, restoring RNAi-activity led to the finding that siRNAs had been raised only against a subset of the usual transcripts, suggesting that particular conditions under which various transposons are expressed had not been met. Missed out were siRNAs against RNAi target(-region)s linked to Ago2. But how would wild-type cells maintain these siRNAs when grown under similar conditions?
In below alignment the region in red on the bottom row corresponds to RBM0 as described in [Elela-2018]. Also note that in the catalytic core (3rd row), the RNAse III specific D3 has not been conserved in Rde3, which begs the question what enzymatic activity this protein has. Rnt1 functions as a dimer; its domain structure is shown in the inset at the top [Elela-2018].
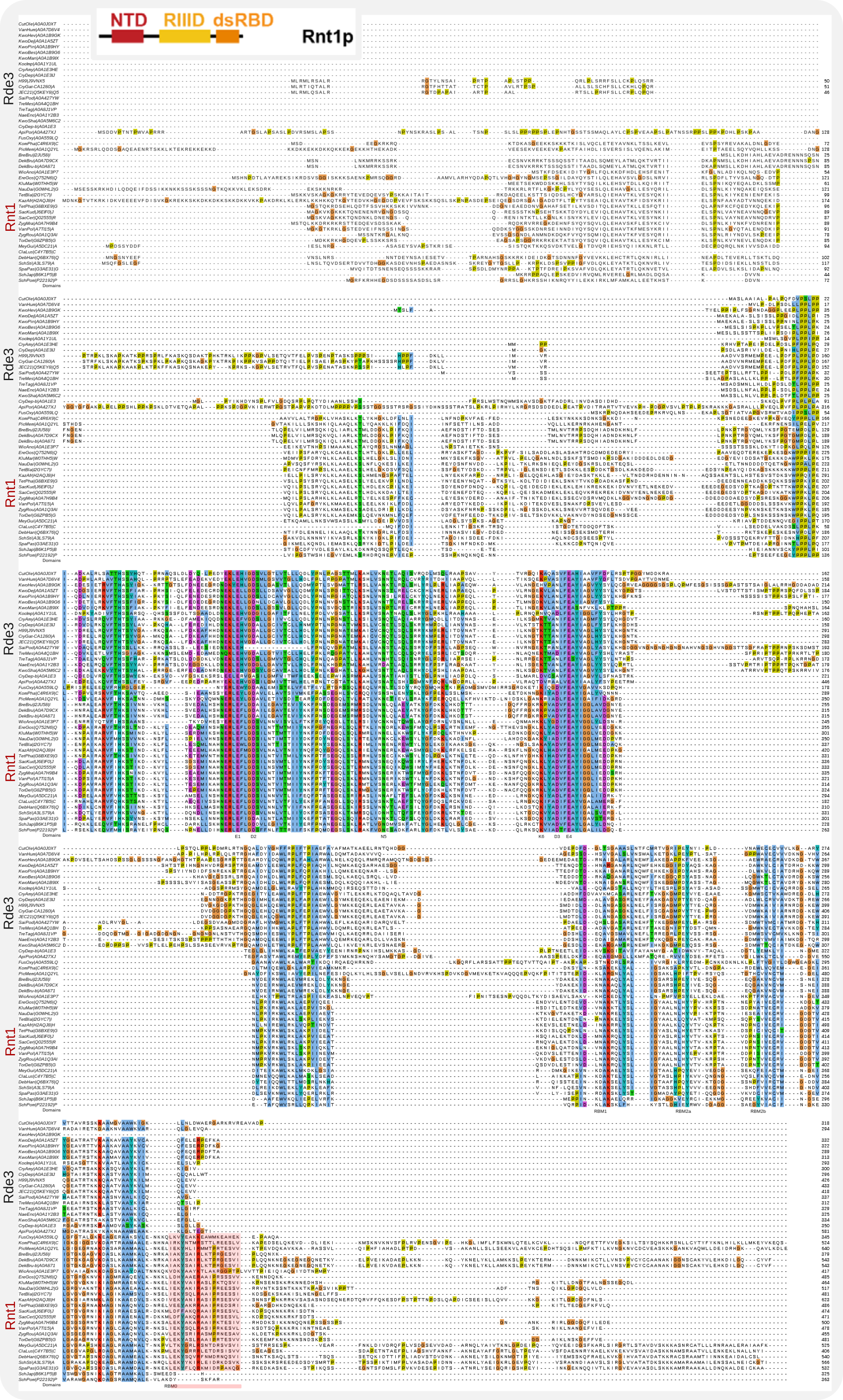
Alignment of Rde3/Rnt1 homologues with catalytic core and RBMs annotated¶
In mammalian cells “inefficient SRP interaction with a nascent chain triggers a mRNA quality control pathway” with AGO2 occupying the leader when SRP54 cannot recognize the exposed peptide [Karamyshev-2013]. Linked to the pioneer round of translation, CBC binding to SRP-RNA ensures translational repression of RNC–SRP until its targeting to the ER has completed [Park-2021].
[Parambil-2023] describes a model and findings where poorly translating transcripts are detetcted/enriched on monosomes during a pioneering round of translation involving CBP (Cap-binding proteins) and EJC (Exon-junction complex) proteins of which some co-purify with SMEDWI-1, like CBP80, ALY/REF and POLD3P/SKAR. These proteins, like Eif4A3, Y14, MAGOH, and PABPN are not present on polysomes during normal translation, which is also the case for SMEDWI-1. Thus not stalling by collapsing ribosomes on polysomes but a translation defect at an earlier stage selects target transcripts in stem-cells of planarian Schmidtea mediterranea. SMEDWI-1 generates sense piRNAs directly from these transcripts, which also relies on the nuclease Zucchini that is bound to the mitochondrial outer membrane. In Cryptococcus there are no piRNAs and no obvious homologues for Zucchini, while siRNAs appear to be sense plus antisense. Still, the pioneering round of translation is coupled to nuclear transport of spliced mRNA given the EJC factors involved; these are conserved in Cryptococcus. In yeast, a kind of monosomes are formed prior to the final maturation step of 18S in the cytosol [Lebaron-2012] and pioneer rounds of translation linked to NMD are occurring on both CBP80/EJC bound mRNA exported from the nucleus and on eIF4E-bound mRNA translated in the cytosol [Gao-2005]. If similar translation checks happen in Cryptococcus, problematic transcripts could stall ribosomes and induce RNAi both prior to and during the modeled events (CBC-bound mRNAs can be occupied by more than one ribosome although not by as many as eIF4E-bound mRNA).